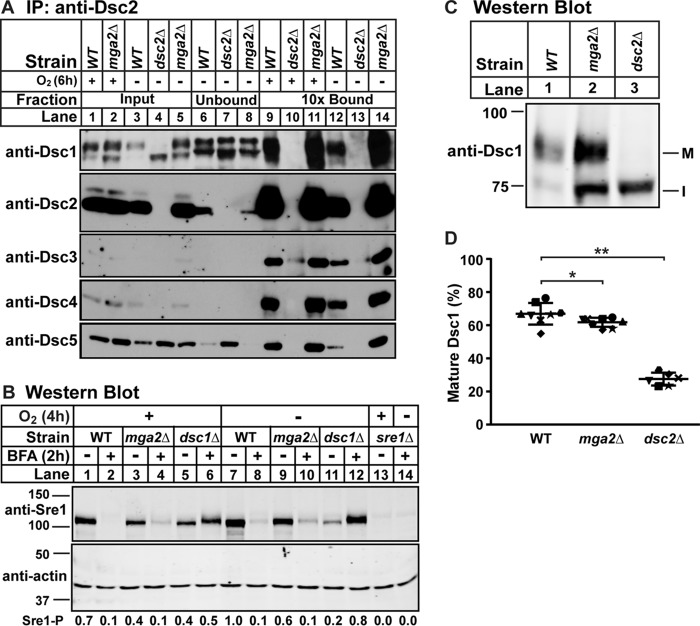
FIGURE 4.
Dsc E3 ligase is functional in mga2Δ cells. A, digitonin-solubilized membrane protein was prepared from WT, mga2Δ, or dsc2Δ cells grown in the presence or absence of oxygen for 6 h, and the Dsc E3 ligase complex was immunoprecipitated (IP) with anti-Dsc2 polyclonal IgG as described under “Experimental Procedures.” Equal quantities of input, unbound, and 10-fold bound fractions were analyzed by immunoblotting using HRP-conjugated antibodies against Dsc1, Dsc2, Dsc3, Dsc4, and Dsc5 (imaged by film). The blot is representative of three biological replicates. B, Western blot, probed with polyclonal anti-Sre1 IgG and monoclonal anti-actin (for loading) and imaged by LI-COR Biosciences Odyssey CLx, of lysates from WT, mga2Δ, dsc1Δ, or sre1Δ yeast grown for 2 h in the presence or absence of oxygen and then treated with BFA (100 μg/ml) or EtOH vehicle for another 2 h in the presence or absence of oxygen. Quantification of Sre1 precursor (Sre1-P) normalized to WT vehicle in the absence of oxygen (lane 7) is shown below. The blot is representative of two biological replicates. C, Western blot, probed with polyclonal anti-Dsc1 IgG and imaged by LI-COR Biosciences Odyssey CLx, of Nonidet P-40-solubilized membrane protein from WT, mga2Δ, or dsc2Δ cells grown in the presence of oxygen. M and I indicate mature and intermediate glycosylated forms, respectively. D, quantification of Dsc1 from C of seven replicates. The quantity of the mature form was divided by total Dsc1 signal for percent mature, allowing comparison between lanes and blots. Error bars are 1 S.D. (*, p < 0.05; **, p < 0.01 by two-tailed Student's t test).