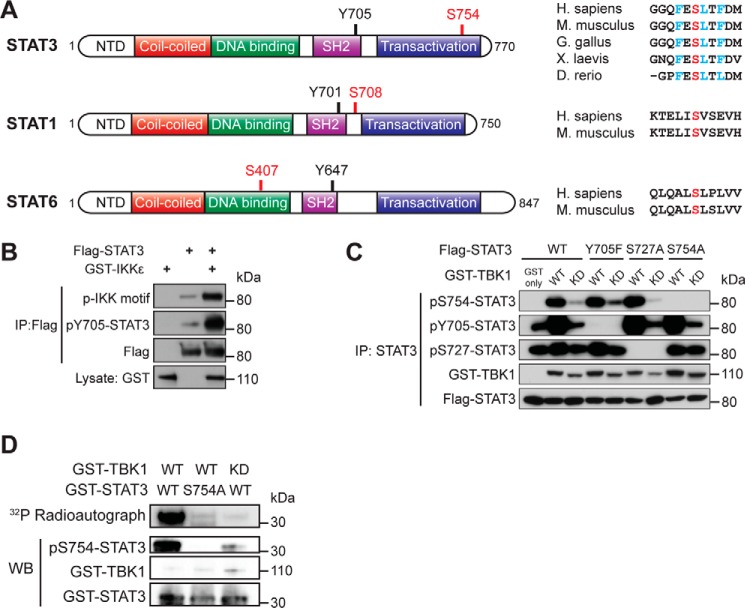
FIGURE 1.
IKKϵ and TBK1 induce STAT3 phosphorylation at Ser754. A, a schematic figure showing the domain structure of STAT3 and the location of Ser754. Sequences of STAT3 from several vertebrates were aligned to compare the homology, with residues critical for TBK/IKKϵ substrate recognition marked in blue and Ser754 marked in red. Also shown are the domain structure of STAT1 and STAT6, with previously identified IKKϵ/TBK1 target serine residues marked in red and the sequence alignments shown on the right. NTD, N-terminal domain. B, FLAG-tagged STAT3 (3 μg) was co-transfected with GST-IKKϵ (3 μg) into HEK293T cells. STAT3 was immunoprecipitated (IP) and blotted with an IKK substrate motif antibody. C, FLAG-tagged wild-type, Y705F, S727A, or S754A STAT3 (3 μg) were co-transfected with wild-type or K38A (kinase-dead; KD) GST-TBK1 (3 μg) into HEK293T cells. STAT3 was immunoprecipitated and blotted with phosphorylation-specific antibodies to detect Ser754, Tyr705, and Ser727 phosphorylation. D, in vitro kinase assay using the GST-tagged C terminus of STAT3 and GST-TBK1 purified from HEK293T as described under “Experimental Procedures.” The mixture was resolved by SDS-PAGE and blotted (WB) with GST and Ser(P)754-STAT3 antibodies. Phosphorylation of STAT3 was also detected by autoradiography. Data in B–D are representative of four, three, and two independent experiments, respectively.