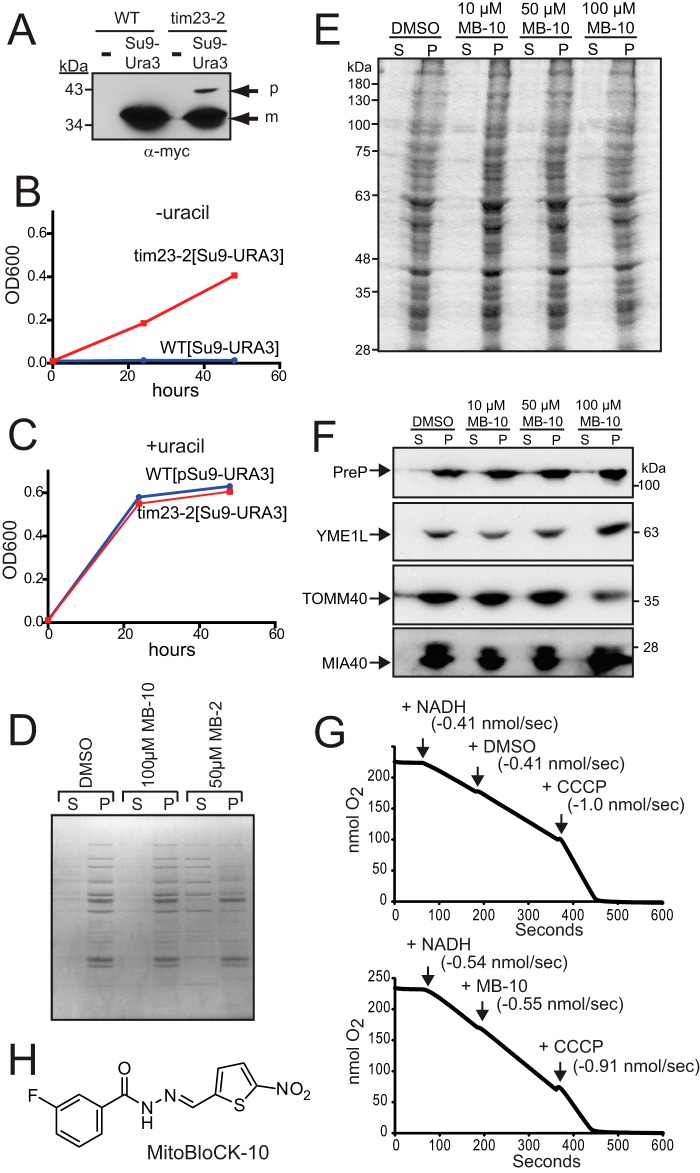
FIGURE 1.
MB-10 is a potential attenuator of protein import into mitochondria. A, a plasmid expressing Su9-Ura3-myc was integrated at the LEU2 locus in WT and tim23-2 strains, and the strains were grown at 30 °C. A whole cell lysate from WT and the tim23-2 mutant, either without (−) or with [Su9-Ura3], was separated by SDS-PAGE followed by immunoblotting with an anti-Myc antibody. p, precursor; m, mature. B, growth analysis of the WT and tim23-2 mutant with integrated [Su9-URA3] in synthetic dextrose medium in the absence of uracil. Growth was measured based on the density at A600 (OD600). C, as in B, in the presence of uracil. D, 1% DMSO, 100 μm MB-10, or 50 μm MB-2 was added to purified 100 μg/ml WT yeast mitochondria for 30 min at 25 °C in import buffer, and released proteins (S) were separated from mitochondria (P) by centrifugation. The proteins were visualized by Coomassie staining. E, as in B, 1% DMSO or the indicated concentration of MB-10 was added to purified mitochondria from HEK293T cells followed by Coomassie staining. F, as in E, the release of proteins was detected by immunoblotting; proteins included PreP (matrix), YME1L (inner membrane), TOMM40 (outer membrane), and MIA40 (intermembrane space). G, respiration measurements were performed with a Clark-type oxygen electrode using 100 μg/ml WT yeast mitochondria in the presence of 1% DMSO or MB-10. Respiration was initiated with NADH addition. 100 μm MB-10 (with DMSO at a 1% final concentration) or 1% DMSO was added once steady-state respiration was established. As a control, CCCP was added to uncouple the mitochondria. The respiration rate for each treatment has been included. H, the structure of MitoBloCK-10 (MB-10).