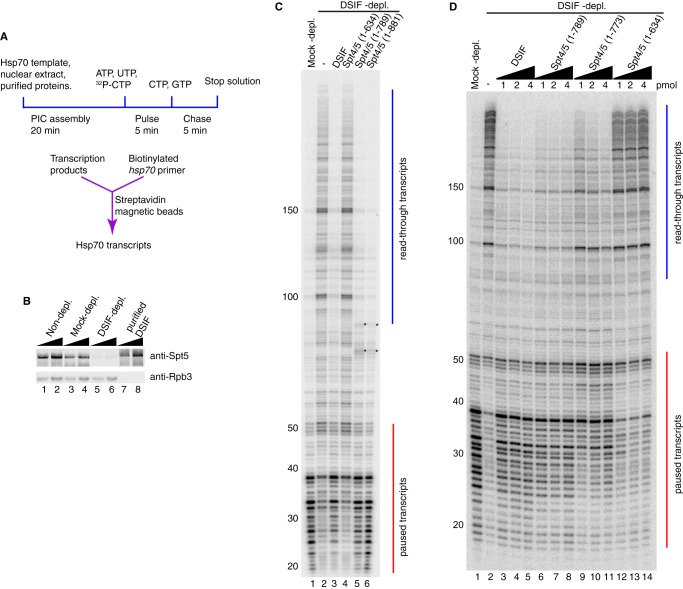
FIGURE 2.
In vitro transcription with different versions of DSIF reveals a region in Spt5 important for promoter-proximal pausing. A, diagram of the in vitro transcription assay. Normal and mutant DSIF proteins were added to nuclear extracts depleted of endogenous DSIF and then incubated with a plasmid containing the hsp70 promoter. Transcripts were radiolabeled by a pulse-chase procedure and purified by hybridizing to biotinylated hsp70 oligonucleotides that were then captured by streptavidin magnetic beads. Hsp70 transcripts were analyzed on a 10% polyacrylamide gel containing 8 m urea. B, Western blotting analysis of nuclear extracts and recombinant DSIF probed with antiserum against Spt5. Lanes 1–6 show two amounts each of non-depleted extract, mock-depleted extract, and DSIF-depleted extract, corresponding to one-eighth or one-fourth of nuclear extract used in each in vitro transcription reaction. Lanes 7 and 8 correspond to 0.125 and 0.25 pmol of recombinant DSIF. Rpb3 is a subunit of RNA polymerase II. C and D, DSIF with different Spt5 mutations was compared with normal DSIF for pausing activity. Red vertical lines from +20 to +50 indicate paused transcripts. Blue vertical lines from +100 and beyond indicate read-through transcripts. C, truncation mutants Spt4/5(1–881), Spt4/5(1–789), and Spt4/5(1–635) were first tested. Spt4/5(1–881) and Spt4/5(1–789) restored pausing similarly as normal DSIF. Spt4/5(1–635) failed to restore pausing. The asterisks in lanes 5 and 6 indicate contaminating radiolabeled nucleic acids generated by the extract independently of Pol II (7). The results shown in C are representative of three independent experiments. D, 1, 2, and 4 pmol of Spt4/5(1–789), Spt4/5(1–773), and Spt4/5(1–635) were further tested. The amount of protein used in each reaction is shown above each lane. Spt4/5(1–789) restored pausing. Spt4/5(1–635) failed to restore pausing. Spt4/5(1–773) partially restored pausing and shifted the pause sites downstream. The results shown in D are representative of two independent experiments.