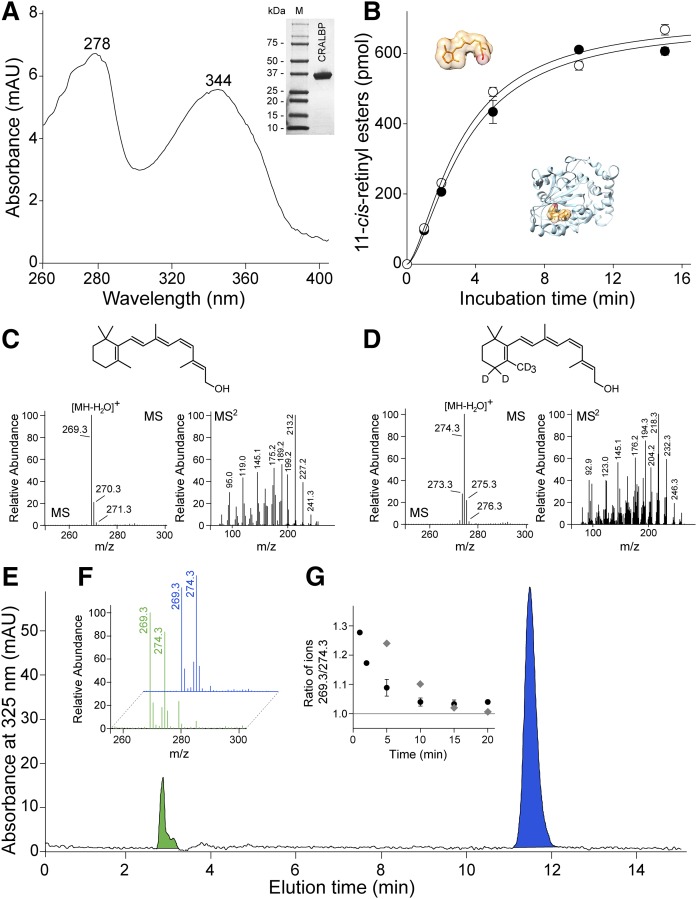
Fig. 3.
AWAT2-dependent esterification of 11-cis-retinol in the presence of holo-CRALBP. A: The apo-CRALBP expressed in E. coli cells was purified to homogeneity (inset) and preincubated with 11-cis-retinol to form holo-protein. After repurification, the efficiency of ligand binding was verified by recording UV/Vis absorbance spectra. The spectrum indicates two maxima at 278 nm corresponding to the protein scaffold and at 344 nm that is representative of bound 11-cis-retinol. B: The time course of 11-cis-retinol esterification in the presence of either free (open circle) or CRALBP-bound (filled circle) substrate. C, D: Verification of the isotopic composition of 11-cis-retinol (C) and its deuterated form (D). C: The MS spectrum of unlabeled retinoid reveals the main isotopic peak at m/z 269.3 that corresponds to ionization-driven loss of water from the parent ion. The conforming tandem spectrum (MS2) shows the fragmentation pattern of the retinoid moiety. D: The main peak for heavy atom-labeled 11-cis-retinol is at m/z 274.3 that indicates five deuterium atoms incorporated into the molecule. The ion at m/z 274.3 that corresponds to the molecules labeled with only four heavy atoms represents less than 20% of the mixture. Importantly, the unlabeled retinoid was not detectable. E: HPLC separation of 11-cis-retinol (blue peak) and its esterified form (green peak) after 2 min incubation of AWAT2 with holo-CRALBP and free 5D-11-cis-retinol. The isotopic composition of the substrates (blue) and products (green) are shown in (F), whereas (G) represents temporal changes in the ratio between labeled and unlabeled 11-cis-retinyl esters during the enzymatic reaction (filled circle) and the time course of ligand exchange between holo-CRALBP and free 11-cis-retinol (gray diamonds).