Figure 2.
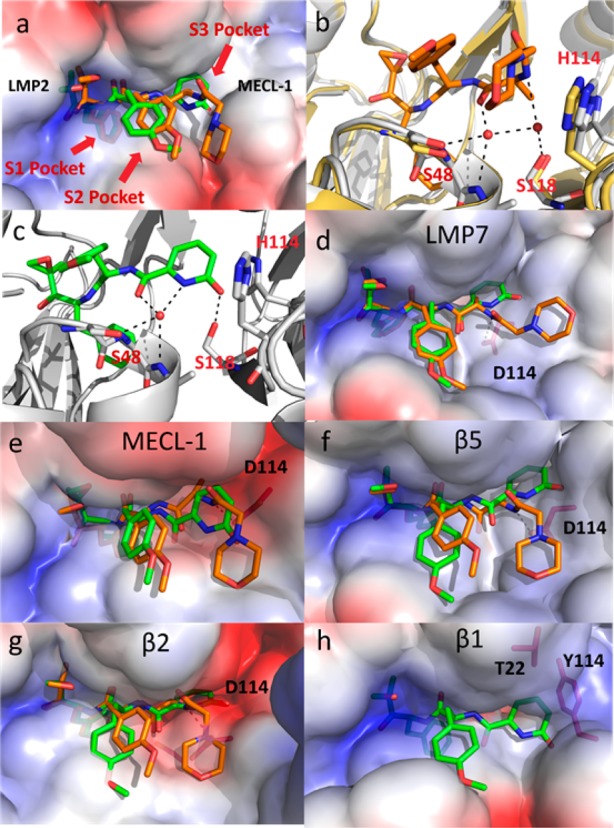
(a) ONX 0914 (orange) and 10 (green) modeled within the human LMP2 structure (van der Waals surfaces). The phenylalanine substituent of the peptide backbone of these inhibitors is buried below the surface within the S1 pocket. The shallow S3 pocket created at the interface of LMP2 and MECl-1 is occupied by the 2-pyridone of 10. (b) ONX 0914 (orange) modeled within the human LMP2 structure (gray) with mouse β1 (yellow, PDB code: 3UNF) overlaid. Based upon the high degree of sequence homology between mouse β1 and human LMP2, a similar water network is assumed to be present in both structures. (c) The 2-pyridone of 10 (green) displaces a water molecule within the S3 pocket of LMP2 (gray) while maintaining a hydrogen bonding network with S48 and S118. (d) Comparison of ONX 0914 (orange) and 10 (green) modeled in the human LMP7. (e) ONX 0914 (orange) and 10 (green) modeled in human MECL-1. (f) ONX0914 (orange) and 10 (green) modeled in human β5. (g) ONX 0914 (orange) and 10 (green) modeled in human β2. (h) Compound 10 (green) modeled in LMP2, overlaid on the human β1 model. The H114Y and A22T substitutions from LMP2 to β1 occupy the space in which the 2-pyridone would reside.
