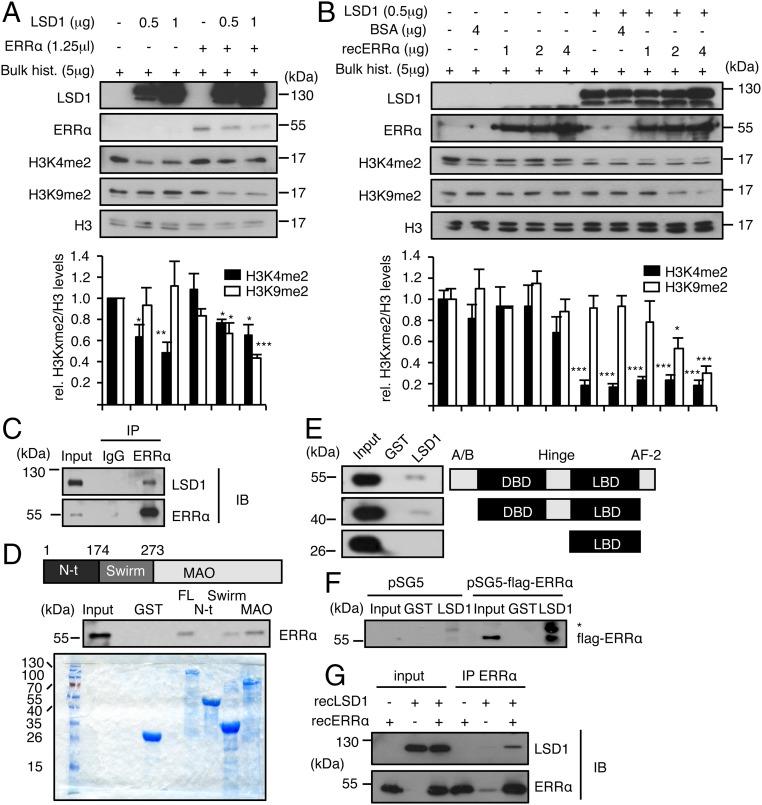
Fig. 3.
ERRα interacts with LSD1 and promotes H3K9 demethylase activity. (A and B) Bulk histones were incubated with recombinant LSD1 in demethylation buffer. Reactions were supplemented with ERRα translated in reticulocyte lysates (A) or recombinant ERRα protein (B). Unprogramed reticulocyte lysate (A) or BSA (B) was used as controls in the absence of ERRα. Levels of H3K4me2, H3K9me2, and H3 were analyzed by Western blot. Quantifications of histone marks were determined using ImageJ software and are expressed relative to H3 and to control conditions. Bars represent the mean ± SEM of four independent experiments. Significance is shown relative to control. *P < 0.05; **P < 0.01; ***P < 0.005. (C) Coimmunoprecipitation of endogenous proteins in MDA-MB231 cells with anti-LSD1 or anti-ERRα antibodies and rabbit IgG used as a control. IB, immunoblotting; IP, immunoprecipitation. (D) Pull-down assay using the indicated GST-fused LSD1 derivatives and nuclear extract from HeLa cells. FL, full-length; MAO, MonoAmine Oxidase domain; N-ter, N-terminal domain. Coomassie blue staining below shows the expression of GST-fused proteins used. (E) Pull-down experiment using GST-LSD1 (full-length) and nuclear extract from HeLa cells transfected with the indicated flag-tagged ERRα derivatives. (F) In vitro interaction assay using GST-LSD1 (full-length) and in vitro translated flagged ERRα or empty vector (pSG5). Western blots were probed with flag antibody. *, nonspecific band. (G) Recombinant LSD1 and recombinant ERRα were incubated in demethylation buffer. Immunoprecipitation was then performed using anti-ERRα antibody. Immunoblots (IBs) were probed with the indicated antibodies. Shown are 20% inputs.