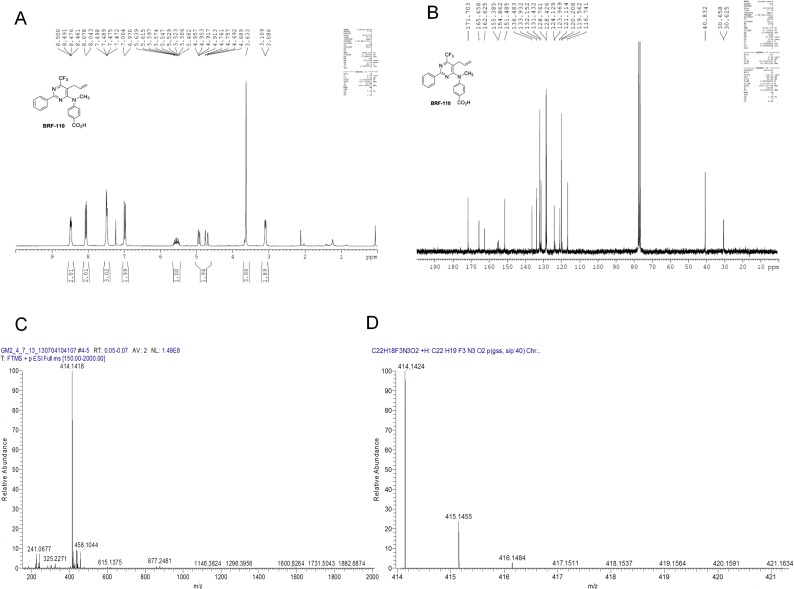
Fig. S2.
Characterization data for BRF110 4-((5-allyl-2-phenyl-6-(trifluoromethyl)pyrimidin-4-yl)(methyl)amino)benzoic acid. MW = 413.1351, cLogP = 6.14 (for neutral), cLogD = 3.33 at pH 7.4, PSA = 66.32 Å2, HBD = 1, logS = −3.60, log1/S for neutral comp = 6.41, lolubility (S) = 0.10 g/L at pH 7.4. (A) 1H NMR of BRF110. 1H NMR spectra were recorded on a 250-MHz Bruker Avance FT-NMR spectrometer. 1H NMR (250 MHz, CDCl3) δ 8.47 (m, 2 H), 8.05 (d, 2 H, J = 8.8 Hz), 7.47 (m, 3 H), 6.98 (d, 2 H, J = 8.8 Hz), 5.55 (m, 1 H), 4.93 (dd, 1 H, J = 1.0, 10.0 Hz), 4.72 (dd, J = 0.9, 17.1 Hz), 3.62 (s, 3 H), 3.08 (d, 2 H, J = 5.8 Hz). (B) 13C NMR of BRF110. 13C NMR spectra were recorded at 62.9 MHz. Distortionless enhancement by polarization transfer spectra [DEPT (135)] were recorded at 62.9 MHz. 13C NMR and [DEPT (135)] data are combined and represented as follows: chemical shift, carbon type obtained from [DEPT (135)] experiments. Chemical shifts are reported in ppm relative to solvent signal. Multiplicity is indicated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad; dd, doublet of doublets; ddd, doublet of doublets of doublets. 13C NMR (62.9 MHz, CDCl3) δ 171.7 (C), 165.6 (C), 162.6 (C), 155.1 (C, q, JC–F = 32.9 Hz), 151.5 (C), 136.5 (C), 133.9 (CH), 132.1 (CH), 131.4 (CH), 128.8 (CH), 128.4 (CH), 124.1 (C), 121.8 (C, d, JC–F = 277.2 Hz), 121.1 (C), 120.2 (CH), 116.7 (CH2), 40.8 (CH3), 30.6 (CH2, d, JC–F = 2.1 Hz). (C) HRMS of BRF110. (D) Elemental composition search on mass 414.1418 (isotopes O16, C12, H1, N14, F19), m/z 414.1418; theoretical mass, 414.1424; delta (ppm), −1.52; RDB equivalent, 13.5; composition, C22 H19 O2 N3 F3.