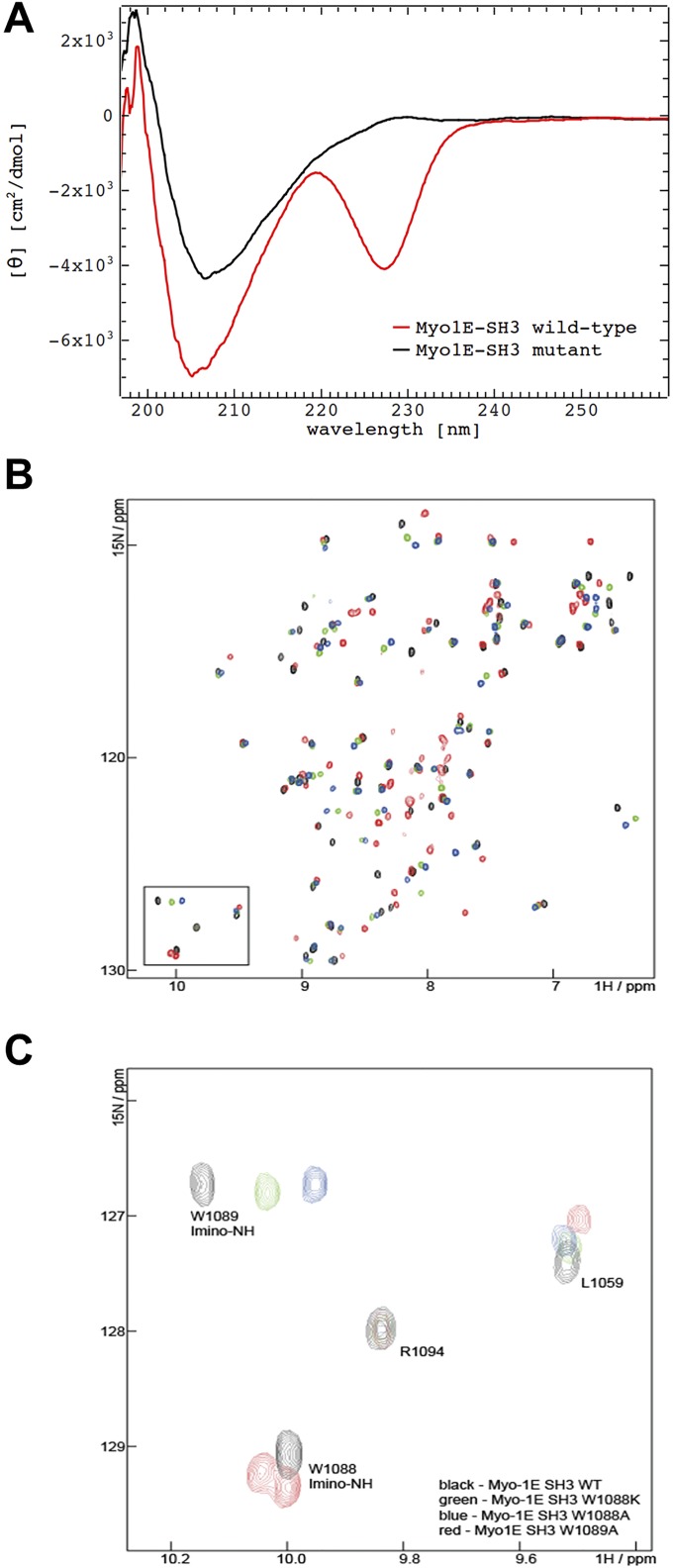
Fig. S3.
Assessment of folding of recombinant MYO1E SH3 domains by circular dichroism and NMR. (A) Circular dichroism spectra of recombinant WT and W1088K MYO1E SH3 domains (after GST-tag removal). Results indicate folded proteins. (B and C) Assignment of the tryptophane side-chain imine peaks: NMR spectra of MYO1E SH3 WT (black), W1088A (blue), 1088K (green), and 1089A (red) in the absence of peptide. (B) Overlay of the spectra in the imino region demonstrates that the bottom imino peak is absent in the spectra of W1088A and W1088K, thus indicating that this peak corresponds to W1088. Vice versa, the top imino peak is absent in W1089A. In this mutant, the W1088A peak displays peak splitting, which can be attributed to the poorer spectral quality of this mutant. (C) Overlay of the whole spectra of Myo-1E SH3 WT and tryptophane mutants. The spectra of W1088A and W1088K display well-dispersed peaks that partially overlap with the WT protein. This finding indicates that these mutants are well folded and adopt the same fold as the WT protein. The spectrum of W1089A (red) displays poorer spectral quality and some peak splitting.