Figure 2.
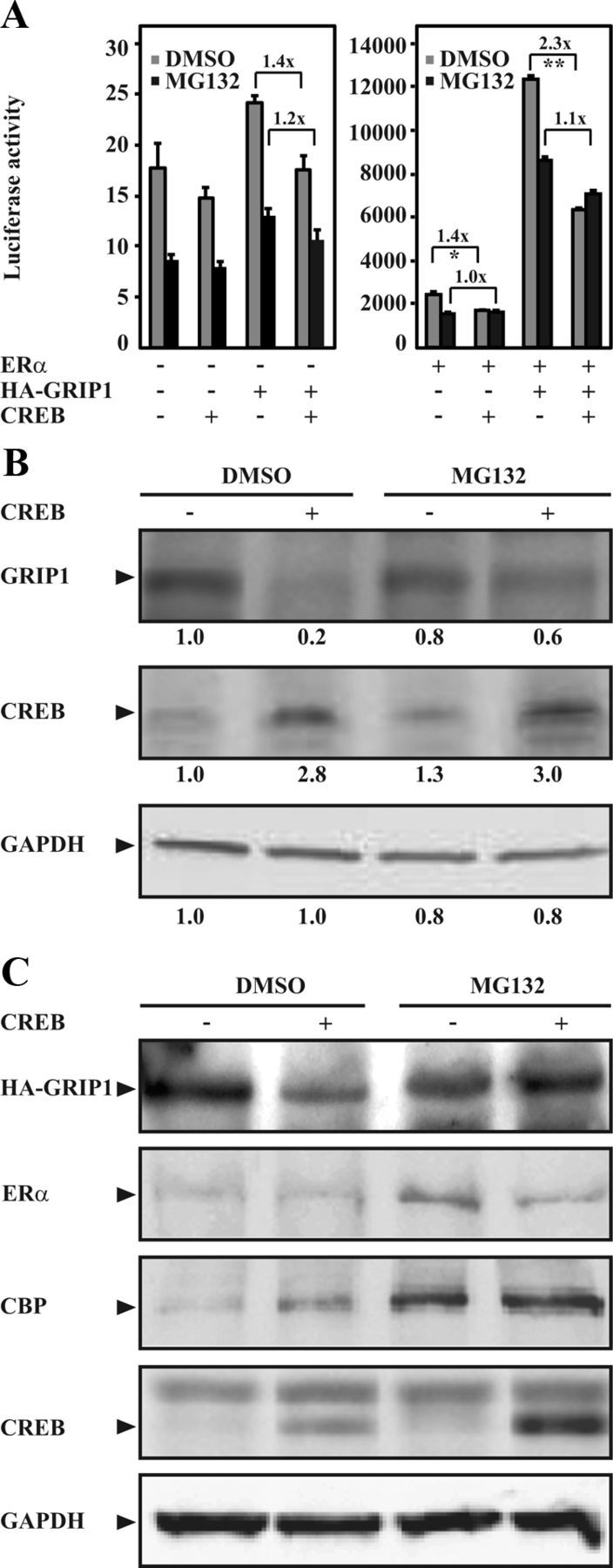
CREB reduces GRIP1 protein level through proteasomal protein degradation. A, COS-1 cells seeded in phenol red-free DMEM supplemented with charcoal-stripped FBS (5%) were transfected with expression plasmids encoding human ERα (0.1 μg), HA-GRIP1 (1.0 μg), and CREB (0.1 μg) together with the ERE-TATA-luc reporter construct (1.1 μg). Twenty-four hours after transfection, cells were treated with 17β-estradiol (E2, 0.1μM). Transfected cells were also treated with vehicle (DMSO) or MG132 (1μM) for 16 hours as indicated in the figure. Luciferase assays were performed 48 hours after transfections. The results are presented as mean ± SD of triplicate transfections from 3 independent experiments (**P < .01; *P < .05, Student's t test). Fold reduction is also presented. B and C, COS-1 cells were transfected with expression plasmid encoding CREB (0.1 μg) alone (B) or together with expression plasmids encoding human ERα (0.1 μg) and HA-GRIP1 (1.0 μg, C) and treated with DMSO or MG132 as in A. Cell lysates were analyzed by Western blotting using anti-TIF2 or anti-HA antibodies for detection of endogenous GRIP1 (B) or overexpressed GRIP1 (HA-GRIP1, C), respectively. The levels of CREB, ERα, CBP, and GAPDH were also examined. Relative protein levels of GRIP1, CREB, and GAPDH (B) were assessed as described in Materials and Methods and indicated below each respective panel.
