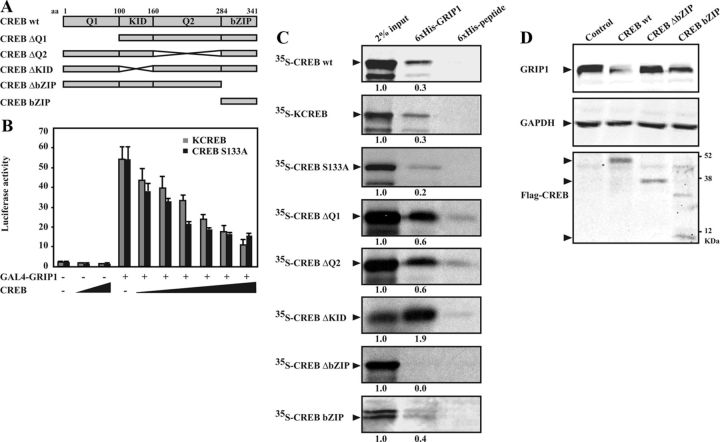
Figure 8.
The bZIP domain of CREB is essential for interaction with and degradation of GRIP1. A, Schematic illustrations of the full-length CREB (CREB wt) with functional domains, and the studied truncated/deletion mutants: CREB ΔQ1 (deletion of Q1), CREB ΔQ2 (deletion of Q2), CREB ΔKID (deletion of KID), CREB ΔbZIP (deletion of bZIP), and CREB bZIP (only bZIP). The approximate aa positions for each functional domain are indicated (uppermost). Q1 and Q2 indicate glutamine-rich domains. B, COS-1 cells were transiently transfected with a GAL4-responsive luciferase reporter construct (GAL4-luc) (1.1 μg) together with expression plasmids encoding GAL4-GRIP1 (1.0 μg) and KCREB or CREB S133A (0.01, 0.02, 0.05, 0.1, 0.5, and 1.0 μg) for 48 hours. Luciferase assays were performed, and the results are presented as mean ± SD of triplicate transfections from a representative experiment. C, In vitro protein interaction assays. [35S]Methionine-labeled CREB proteins (wt, CREB S133A, KCREB, ΔQ1, ΔQ2, ΔKID, ΔbZIP, and bZIP) were incubated with recombinant 6xHis-GRIP1 or 6xHis-peptide as described in Materials and Methods. Interaction complexes were pulled down using Ni-Sepharose beads and resolved by SDS-PAGE followed by autoradiography. Two percent of input was also included. Ratios between densitometries of input and interaction band are indicated below each panel. D, COS-1 cells were transfected with expression plasmids encoding HA-GRIP1 (2.0 μg) and Flag-tagged CREB wt, ΔbZIP, or bZIP (0.5 μg). Cell lysates were analyzed by Western blotting using anti-HA (GRIP1), anti-Flag (Flag-CREB), and anti-GAPDH antibodies.