Fig. 6.
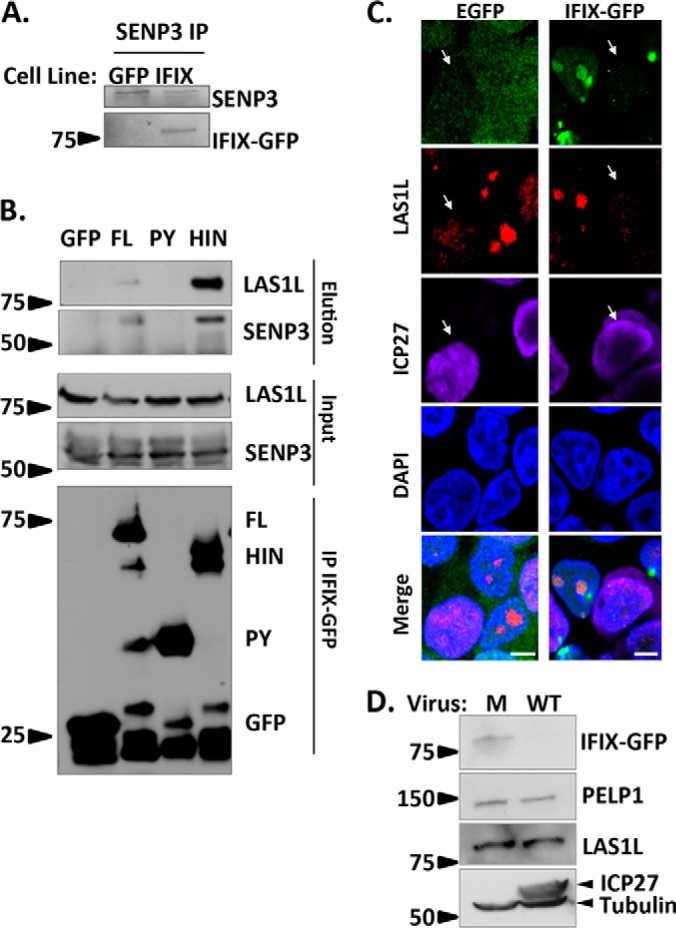
HIN domain of IFIX mediates its interaction with the 5FMC complex. A, validating IFIX co-interaction with SENP3 by reciprocal IP. B, IFIX interacts with 5FMC components LAS1L and SENP3 through its HIN domain. Forward IPs using GFP antibody were performed in cells transfected with IFIX constructs full-length (FL), IFIX Pyrin domain (PY), and IFIX HIN200 domain (HIN) in pEGFP. Inputs (1.5%), elutions (20%), and isolated IFIX constructs (IP, 20%) were blotted for LAS1L, SENP3, and GFP. C, IF microscopy in IFIX-GFP and EGFP control 293 cells showing a redistribution of 5FMC protein LAS1L in infected cells (white arrows), m.o.i.: 5, 4 hpi. Co-localization of IFIX and LAS1L is pronounced in uninfected cells (for PELP1, see supplemental Fig. S3). D, Levels of PELP1 and LAS1L are not reduced during HSV-1 infection. PELP1 and LAS1L levels were monitored by western blotting at 6hpi. ICP27 is marker for infection. M, mock. Microscopy images were taken at ×60 oil objective. Bar, 5 μm.
