Abstract
Background
Approximately 40-45% of youth with anxiety disorders do not achieve remission (or a substantial reduction in symptoms) following treatment, highlighting the need to identify predictors of treatment response. Given the well-established link between attentional biases and anxiety disorders in youth and adults, the current study examined the neural correlates of directing attention toward and away from emotional faces in relation to pediatric anxiety treatment response.
Method
Prior to beginning treatment with the selective serotonin reuptake inhibitor (SSRI) sertraline or cognitive behavior therapy (CBT), 37 youth (age 7-19 years) with generalized and/or social anxiety disorder completed a task with conditions that manipulated whether participants were instructed to match emotional faces (explicit emotion processing) or match shapes in the context of emotional face distractors (implicit emotion processing) during functional magnetic resonance imaging.
Results
Results revealed that reduced activation in superior frontal gyrus (SFG), encompassing the dorsal anterior cingulate cortex (ACC) and dorsomedial prefrontal cortex (PFC), during implicit processing of emotional faces predicted a greater reduction in anxiety severity pre-to-post treatment. Post-hoc analyses indicated that effects were not significantly moderated by type of treatment or anxiety type.
Conclusions
Findings suggest that less recruitment of SFG, including the dorsal ACC and dorsomedial PFC, during implicit emotion processing predicts a greater reduction in youth anxiety symptoms pre-to-post treatment. Youth who exhibit reduced activation in these areas while matching shapes in the context of emotional face distractors may have more to gain from CBT and SSRI treatment due to preexisting deficits in attentional control. These findings suggest neuroimaging may be a useful tool for predicting which youth are most likely to benefit from anxiety treatment.
Keywords: Pediatric anxiety, treatment response, neuroimaging, attention, emotion processing
Introduction
Pediatric anxiety disorders, affecting approximately 10%-15% of children and adolescents (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003), are one of the most common classes of psychological disorders in youth and are associated with impairment in social and academic functioning, and increased risk of multiple disorders later in life (Mychailyszyn, Mendez, & Kendall, 2010). Although pediatric anxiety can be treated effectively through the use of cognitive behavioral therapy (CBT) and pharmacological treatments (i.e., selective serotonin reuptake inhibitors [SSRIs]), approximately 40-45% of youth do not achieve remission or a substantial reduction in symptoms (Mohatt, Bennett, & Walkup, 2014). Identifying predictors of outcome may allow clinicians to identify youth at risk for poorer outcomes, and help guide patients towards those treatments with the highest likelihood of success.
Cognitive models of anxiety suggest that individuals’ characteristic ways of attending to threatening stimuli contribute to the development, maintenance, and treatment of the disorder (e.g., Clark, Beck, & Alford, 1999). Supporting these models, studies demonstrate that anxiety disorders in children and adults are characterized by both initial orienting of attention to threatening (angry or fearful) stimuli, and difficultly diverting attention from threatening stimuli (e.g., Bishop, 2007; Pine, 2007; Bar-Haim et al., 2007). Studies investigating the neural correlates of these attentional responses in anxiety highlight overactivation of regions associated with the detection of threat, such as the amygdala, and altered top-down control by regions associated with goal-directed attention, such as the lateral prefrontal cortex (PFC) and anterior cingulate cortex (ACC) (Bishop, 2007). Engagement of the ACC in particular appears to be critical in maintaining an appropriate balance between sensitivity to potential threat and carrying out task-relevant cognitive goals (i.e., directing attention away from threat) (Etkin et al., 2006).
Notably, anxious youth (Beesdo et al., 2009; Guyer et al., 2008) and adults (Etkin & Wager, 2007) exhibit increased amygdala reactivity when performing tasks with threatening stimuli and less activation in the ACC while performing tasks that require participants to direct their attention away from emotional stimuli (Bishop, Duncan, Brett, & Lawrence, 2004; Klumpp et al., 2011). Moreover, in a task developed in our lab, we had previously shown that when participants are instructed to match emotional faces (explicit emotion processing) or match shapes in the context of emotional face distractors (implicit emotion processing), both youth (Swartz et al., 2014) and adults (Klumpp, Post, Angstadt, Fitzgerald, & Phan, 2013) with anxiety disorders, compared with healthy controls, exhibit less ACC activation during implicit versus explicit matching.
While these previous studies have generally been consistent in supporting this neuropathophysiological model in anxiety disorders (i.e., enhanced amygdala activation during explicit emotion processing and decreased prefrontal activation when required to direct attention away from emotional stimuli), surprisingly few studies have examined how neural correlates of these processes relate to anxiety treatment outcome. In one study of adults with social anxiety disorder, greater activation of the dorsal ACC during implicit emotion processing (i.e., matching shapes in the context of emotional face distractors) predicted better response to CBT (Klumpp, Fitzgerald, Angstadt, Post, & Phan, 2014). However, only two previous fMRI studies have examined predictors of treatment response in youth with anxiety disorders, and both of these studies focused solely on explicit emotion processing. In one study, greater amygdala activation in response to viewing fearful faces predicted better treatment response among a sample of anxious youth (McClure et al., 2007). More recently, using a different task that probed explicit emotional processing only, we found that greater activation in dorsolateral and ventrolateral PFC while processing threatening faces predicted greater response to CBT and SSRI treatment (Kujawa et al., in press).
The primary aim of the current study was to extend these previous studies by examining neural correlates of both explicit and implicit emotion processing in relation to treatment outcome for pediatric anxiety. This two-site study was modeled after the Child/Adolescent Anxiety Multimodal Study (Compton et al., 2010) in that it included children and adolescents across a large span of development (7-19 years) with primary diagnoses of generalized anxiety disorder (GAD) and/or social anxiety disorder (SAD), two of the most common anxiety disorders in youth (Mohatt et al., 2014), treated with either sertraline (i.e., SSRI) or psychotherapy (i.e., CBT). Prior to treatment, participants completed the Emotional Faces Shifting Attention Task (EFSAT; Klumpp, Angstadt, & Phan, 2012, Klumpp et al., 2013, 2014; Swartz et al., 2014) in which images comprising a trio of geometric shapes (circles, rectangles, triangles) were presented alongside a trio of emotional facial expressions (angry, fearful, happy), and the participant was prompted to match either faces or shapes. The task provides a measure of neural correlates of both explicit and implicit emotional processing, as the instruction to “Match Shapes” requires participants to direct attention away from emotional faces, whereas “Match Faces” requires individuals to direct attention toward emotional faces.
Based on findings that patients with anxiety disorders exhibit less ACC recruitment in the presence of angry, fearful, or happy face distractors (i.e., match shapes > match faces; Klumpp et al., 2013; Swartz et al., 2014), we expected that recruitment of the ACC during implicit emotion processing (Match Shapes) would influence response to treatment. Regarding the direction of this effect, although we previously demonstrated that greater dorsal ACC activation during implicit processing predicts better response to CBT among anxious adults (Klumpp et al., 2014), studies examining attentional biases in children at the behavioral level suggest the opposite pattern. For example, children who experience difficulty diverting their attention away from threat-relevant stimuli, perform better with CBT (Waters, Mogg, & Bradley, 2012). This finding is consistent with studies suggesting that attentional biases improve with anxiety treatment (Pishyar, Harris, & Menzies, 2008), and that the therapeutic effects of anxiety treatment are mediated by early changes in attentional bias (Abend & Bar-Haim 2013). Because of these mixed findings, no specific hypotheses were generated regarding the direction of the ACC response during implicit emotion processing and its relation to treatment response in youth with anxiety. Regarding explicit emotion processing (Match Faces), consistent with the two previous pediatric anxiety treatment outcome studies (cf. Kujawa et al., in press; McClure et al., 2007), we predicted that greater activation of the dorsolateral and ventrolateral PFC and the amygdala would be related to better anxiety treatment response.
Methods
Participants
Participants were part of a pediatric anxiety treatment study at the University of Michigan (UM) and University of Illinois at Chicago (UIC). Participants in the study were youth between the ages of 7 and 19 with primary diagnoses of GAD or social anxiety disorder. The Schedule of Affective Disorders and Schizophrenia for School-Age Children (Kaufman et al., 1997) diagnostic interview was used to assess for diagnoses by Master’s or Doctoral level clinicians (see Kujawa et al., 2015 for more details). Exclusion criteria included history of bipolar disorder, schizophrenia, intellectual disability, pervasive development disorders, current substance use disorders, severe depression, or suicidal ideation. Participants with secondary comorbid anxiety, depressive, or externalizing disorders were included in the study (see Participant Characteristics). Participants were not taking psychotropic medications or in psychotherapy for at least 4 weeks prior to the initial assessment.
A total of 44 anxious youth completed the fMRI Emotional Faces Shifting Attention Task (EFSAT), followed by a minimum of 10 weeks of pharmacotherapy or psychotherapy. Data from 6 participants were excluded for movement during the fMRI (> 3 mm in any one direction across each functional run) and 1 participant was excluded for low accuracy on the task (< 20%), leaving a total sample of 37 youth with pre-treatment fMRI and pre- and post-treatment clinical measures (n=21 who received SSRI treatment; n=16 who received CBT treatment). The included sample was 59.5% female; 73.0% Caucasian, 8.1% African American, 5.4% Asian, and 13.5% multiracial; 10.8% identified as Hispanic/Latino.
Procedure
Procedures were approved by the Institutional Review Boards at both UIC and UM. Participants completed the clinical interview and pre-treatment symptom measures during the initial visit. Participants then completed the fMRI scan (within one week of initial interview). At UM, participants were offered and self-selected treatment with SSRI or CBT. At UIC, participants were initially randomly assigned to receive either an SSRI or CBT, but could opt to switch from SSRI to CBT due to intolerable side effects. SSRI treatment consisted of 12 weeks of sertraline prescribed by a child psychiatrist during medication management sessions, beginning with a dose of 12.5 or 25 mg/day and in a flexible-dosing design increasing on subsequent visits up to 200 mg/day based on tolerability and treatment response. CBT was delivered through weekly 60-minute sessions (up to a maximum of 18 sessions) by a Master’s or Doctoral level therapist. Treatment followed an established manualized CBT intervention (i.e., Coping Cat, C.A.T Project) for pediatric anxiety (Kendall and Hedtke, 2006; Kendall et al., 2002).
Measures
Emotional faces shifting attention task (EFSAT Klumpp, Angstadt, Phan, 2012; Klumpp et al., 2013, 2014; Swartz et al., 2014)
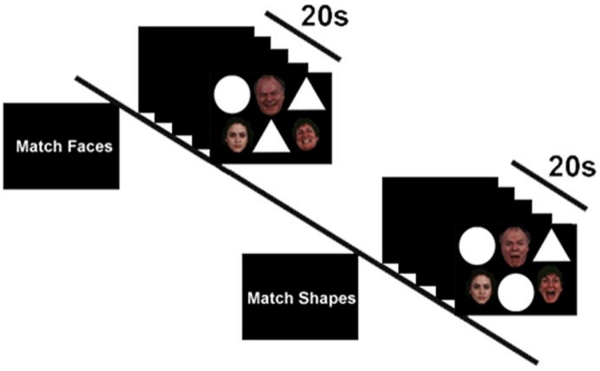
A trial of the EFSAT consists of three faces in a triangular configuration, and three shapes in an upside-down triangular configuration (Figure 1). During the faces condition, participants were instructed to identify which face on the bottom row matched the emotion of the target face on the top. During the shapes condition, participants were instructed to match shapes on the top row with the target shape on the bottom. Participants completed two runs, for a total of 18 faces blocks and 18 shapes blocks with six blocks of each condition: match angry faces, fear faces, and happy faces, and match shapes with angry faces, fear faces, and happy faces as distractors. Each block began with a 4s cue to either ‘match faces’ or ‘match shapes’ followed by the four sequential matching trials, each lasting 4s.
Figure 1.
Schematic of exemplar ‘Match Faces’ and ‘Match Shapes’ blocks in the Emotional Faces Shifting Attention Task (EFSAT). Trials were presented in block format and participants were instructed at the beginning of each block to either match faces or match shapes for that block. The match faces condition requires attending to the emotional faces whereas the match shapes condition requires performing the shape-matching task in the context of emotional face distractors.
fMRI data acquisition and processing
MRI data were collected on 3 Tesla GE scanners with 8-channel head coils at both sites. At UM, functional data were collected with a gradient-echo reverse spiral acquisition with the following parameters: repetition time (TR) = 2s, echo time (TE) = 30ms, flip angle = 90°, = field of view (FOV) = 22 × 22 cm, acquisition matrix 64 × 64, 3-mm slice thickness, and 43 axial slices. At UIC, functional data were acquired using gradient-echo echo-planar imaging (EPI) sequence with the following parameters: TR = 2s, TE = minFull [~25ms], flip angle = 90°, FOV = 22 × 22 cm, acquisition matrix 64 × 64, 3-mm slice thickness, and 44 axial slices.
Functional images were preprocessed in SPM8 (Wellcome Trust Centre for Neuroimaging, http://www.fil.ion.ucl.ac.uk/spm/) for slice timing correction, image normalization, resampling at a 2 × 2 × 2 mm3 voxel size, and 8-mm Gaussian smoothing kernel. Condition effects were modeled at the individual subject level using the general linear model and the nuisance regressors for 6 motion parameters were included to correct for motion artifacts. For each participant, contrast images of brain activity were generated for second-level analysis.
Response to treatment
To assess severity of anxiety symptoms at pre- and post-treatment, participants were administered the Pediatric Anxiety Rating Scale (PARS; Research Units, 2002). The PARS was completed at the initial screening visit and at the final treatment session. Greater PARS change scores (i.e., pre-treatment minus post-treatment) indicate greater response to treatment.
Data analysis
Consistent with previous studies in our lab using the EFSAT task to predict treatment response (Klumpp et al., 2014), a one-sample t-test with change in PARS scores as a covariate of interest was performed in SPM8 for the March Face > Match Shape contrast. To control for methodological and demographic variables, site/scanner (UM or UIC), treatment (SSRI or CBT), and age were included as covariates in the second-level analysis. Based on a priori regions of interest from our hypotheses, we focused on the bilateral amygdala, and the dorsolateral and ventrolateral PFC using the Wake Forest University Pickatlas (WFU Pickatlas) (Maldjian, Laurienti, Kraft, & Burdette, 2003). In order to obtain an ROI for the rostral portion of the ACC, the Anterior Cingulate Region was intersected with the medial frontal region (Swartz et al., 2014) defined by the Automated Anatomical Labeling (AAL) atlas. Activations were deemed as significant using small volume correction (SVC) and family wise error (FWE) corrected p < .05. For completeness, we also examine activation clusters outside a priori ROIs at the whole-brain level using Family Wise Error (FWE) correction at p < .05.
Exploratory analyses were conducted to investigate emotion-specific effects. Specifically, activation (beta weights) from a 10-mm diameter sphere around peak voxels surviving small-volume correction and FWE (ROI regions) for the Match Face > Match Shape contrast were extracted using MarsBar (Brett et al., 2002) and exported into SPSS for the separate emotion contrasts: Match Shape[Angry] > Match Face[Angry], Match Shape[Fear] > Match Face[Fear], and Match Shape[Happy] > Match Face[Happy]. In SPSS, a Generalized Linear Model was then conducted with activation for each emotion type (Angry, Fear, Happy) serving as the within-subject variables and PARS pre-to-post change, site/scanner (UM or UIC), treatment (SSRI or CBT), and age as continuous between-subjects variables. Greenhouse-Geisser corrections were used in cases in which the sphericity assumption was violated and Bonferroni procedures were used to correct for multiple comparisons.
Results
Participant characteristics
With regard to clinical disorders, 67.6% of the sample had current diagnoses of GAD, 59.5% SAD, and 10.8% separation anxiety disorder. In addition, 10.8% had comorbid separation anxiety disorder, 10.8% panic disorder, 2.7% obsessive-compulsive disorder, 18.9% specific phobia, 5.4% depression, and 16.2% ADHD. Anxiety severity (i.e., PARS) decreased following treatment, t(36) = 14.07, p < .001, mean difference = 13.54. With regard to treatment response, 79.1% of the sample responded to treatment and 69.8% showed evidence of remission, as indicated by at least a 35% and 50% decrease in PARS, respectively (Caporino et al., 2013).
Participant characteristics by treatment group are presented in Table 1. CBT and SSRI groups did not significantly differ on PARS, age, or distribution of sex, race, IQ, or primary diagnosis (ps > .13); however, the CBT group completed more sessions than the SSRI group, t(35) = −5.15, p < .001. Study sites did not significantly differ on treatment modality (UM: n= 10 CBT, n = 11 SSRI; UIC: n = 6 CBT, n = 10 SSRI), PARS post-treatment or change, age, number of sessions, or primary diagnoses (ps > .09). Compared to the sample recruited at UM, the UIC sample had greater pre-treatment PARS scores, t(35) = −2.72, p = .01, and included a greater proportion of males, χ2(1) = 5.64, p = .02, and participants who identified as Hispanic/Latino, χ2(1) = 5.89, p = .05.
Table 1.
Participant characteristics by treatment group (selective serotonin reuptake inhibitor [SSRI] or cognitive behavioral therapy [CBT]) and study site (University of Michigan [UM] or University of Illinois at Chicago [UIC]).
| SSRI (n = 21) | CBT (n = 16) | |||
|
| ||||
| Mean | SD | Mean | SD | |
| Age | 14.43 | 2.87 | 14.50 | 3.03 |
| Number of Sessions | 11.90 | 0.44 | 14.87 | 2.60 |
| Pre-treatment PARS | 23.62 | 5.06 | 22.13 | 3.40 |
| Post-treatment PARS | 9.57 | 5.72 | 9.25 | 6.18 |
| PARS change | 14.05 | 6.31 | 12.88 | 5.32 |
| WASI IQ Estimate | 110.41 | 11.24 | 109.38 | 11.98 |
| N | % | N | % | |
| Female | 11 | 52.4 | 11 | 68.8 |
| Primary GAD | 12 | 57.1 | 9 | 56.3 |
| Primary Social Anxiety Disorder | 9 | 42.9 | 7 | 43.8 |
|
| ||||
| UM (n = 21) | UIC (n = 16) | |||
|
| ||||
| Mean | SD | Mean | SD | |
| Age | 14.09 | 2.79 | 14.94 | 3.07 |
| Number of Sessions | 12.81 | 1.94 | 13.69 | 2.63 |
| Pre-treatment PARS | 21.38 | 4.14 | 25.06 | 4.01 |
| Post-treatment PARS | 7.90 | 5.61 | 11.44 | 5.69 |
| PARS change | 13.48 | 6.35 | 13.63 | 5.33 |
| WASI IQ Estimate | 110.76 | 11.34 | 109.00 | 11.83 |
| N | % | N | % | |
| Gender (Female) | 16 | 76.2 | 6 | 37.5 |
| Primary GAD | 13 | 61.9 | 8 | 50.0 |
| Primary Social Anxiety Disorder | 8 | 38.1 | 8 | 50.0 |
Behavioral performance
Behavioral data was lost for 1 participant due to a technical error. To examine the influence of emotion (angry, fear, happy) and condition (shapes versus faces) on accuracy and RT, a repeated-measures ANOVA was conducted. Results revealed a main effect of condition, F(1, 34) = 13.64, p < .001, with accuracy being higher for matching shapes (M = 84.92, SE = 2.10) compared to matching faces (M = 79.22, SE = 2.31) across participants. There was also a main effect of condition for RT, F(1, 34) = 32.83, p < .001, with slower responses when matching faces (M = 1625.06, SE = 98.17) compared to matching shapes (M = 1269.11, SE = 92.15). None of the other main effects or interactions were significant for accuracy or RT (lowest p = .09).
fMRI predictors of treatment response
Results of the ROI one-sample t-test revealed that lower activation of superior frontal gyrus [(12, 54, 20), t-value = 3.47, volume = 2896 mm3, svccorrected p = .03] – a large cluster encompassing the dorsal ACC and ventromedial PFC – during the Match Shape > Match Face contrast predicted a greater reduction in anxiety symptoms pre-to-post treatment. No other regions outside the SFG were associated with change in symptoms for the Match Shape > Match Face contrast within ROIs or for the whole-brain analysis.
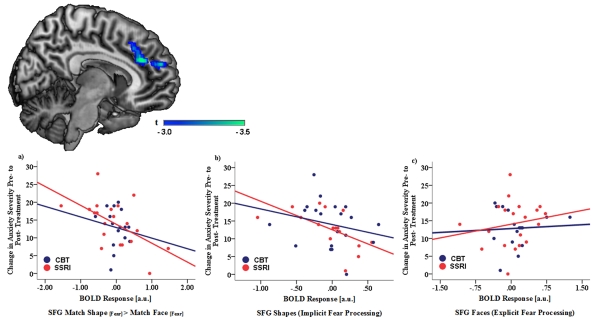
Exploratory analyses were then conducted to examine emotion-specific effects. We extracted beta weights from a spherical ROI around the peak voxel for the SFG cluster (12 54 20) for the Match Shape[Angry] > Match Face[Angry], Match Shape[Fear] > Match Face[Fear], and Match Shape[Happy] > Match Face[Happy] contrasts. A Generalized Linear Model was then conducted with SFG activation for each emotion type (Angry, Fear, Happy) serving as the within-subject variables and PARS pre-to-post change, site/scanner (UM or UIC), treatment (SSRI or CBT), and age as continuous between-subjects variables. Results revealed a significant Emotion × PARS pre-to-post Change interaction for SFG activation, F(2,31) = 3.35, p = .04, np2 = .10. To follow-up this interaction, partial correlations between SFG activation for each emotion type and PARS pre-to-post change were calculated controlling for treatment modality, site, and age. Results revealed a significant negative correlation between PARS pre-to-post change and SFG activation for the Match Shape[Fear] > Match Face[Fear] contrast, r = −.47, p < .01; Figure 2. No significant relations with PARS pre-to-post change were observed for the Match Shape[Angry] > Match Face[Angry] or Match Shape[Happy] > Match Face[Happy] contrasts (lowest p = .12). The correlation between PARS pre-to-post change and SFG activation for the Match Shape[Fear] > Match Face[Fear] contrast remained significant after adjusting for youth’s initial anxiety symptom severity (PARSPreTx), r = −.39, p = .02 and IQ (WASI), r = −.41, p = .02. None of the interactions with participant’s age and gender, study site, and treatment were significant (lowest p = .09). We also examined whether participant’s primary anxiety diagnosis moderated the relation between SFG activation during implicit fear processing and treatment response; none of these analyses were significant (lowest p = .21).
Figure 2.
Exploratory analyses findings showing that reduced superior frontal gyrus, including the dorsal anterior cingulate cortex and dorsomedial prefrontal cortex, activation during processing of shapes in the context of fearful face distractors predicted greater change in anxiety severity, measured via the Pediatric Anxiety Rating Scale, pretreatment to posttreatment. Association between anxiety symptom change, measured via the Pediatric Anxiety Rating Scale, pre-to-post treatment and superior frontal gyrus (SFG) activation (mean blood-oxygen-level-dependent parameter estimates; a.u. = arbitrary units) during a) Match Shapes [Fear] > Match Faces [Fear], b) Match Shape [Fear] > Baseline (Implicit Processing), c) Match Faces [Fear] > Baseline (Explicit Processing).
Finally, because the shapes > faces contrast can be interpreted as decreased activation when matching shapes (implicit emotion processing) or increased activation when matching faces (explicit emotion processing), we extracted beta weights from a spherical ROI around the peak voxel for the SFG for Match Shape[Fear] > Baseline (activation across all conditions) and Match Face[Fear] > Baseline contrasts. Partial correlations in SPSS between brain activation and PARS pre-to-post change were then calculated controlling for age, treatment modality and site. As shown in Figure 2, SFG activation during implicit fear processing was negatively correlated with PARS pre-to-post change (r = −.38, p = .02); however, the Match Face[Fear] > Baseline contrast was not related to PARS change (r = .12, p = .48).
Discussion
The current study used fMRI to examine potential brain predictors of treatment response for pediatric anxiety. Given the well-established link between attentional biases and anxiety disorders, (e.g., Bishop, 2007; Pine, 2007; Bar-Haim et al., 2007), the current study examined the neural correlates of directing attention toward (explicit emotion processing) and away (implicit emotion processing) from emotional stimuli in relation to pediatric anxiety treatment response. Results revealed that reduced activation in SFG, encompassing the dorsal ACC and dorsomedial PFC, during implicit emotion processing predicted a greater reduction in anxiety severity pre- to post-treatment. Exploratory analyses revealed that this effect appeared to be driven by implicit fear processing, and was not observed for matching faces in the context of happy or angry face distractors. We found no evidence for these effects being moderated by type of treatment or primary diagnosis, suggesting similar effects across SSRI and CBT interventions, as well as diagnoses of GAD and social anxiety disorder.
The ACC plays a crucial role in attention-emotion processes (Bush et al., 2000; Carter et al., 1998), and the dorsal ACC, in particular, has been linked to conflict monitoring and cognitive appraisal processes (Etkin, Egner, & Kandel, 2011). In children and adults with anxiety, the ACC has been linked to ability to direct attention away from stimuli when threatening material is presented (e.g., Bishop et al., 2004; Klumpp et al., 2011, 2012; Swartz et al., 2014). Thus, children who recruit less dorsal ACC during attempts to process shapes when emotional stimuli are presented may have more to gain from CBT and SSRI treatment due to preexisting deficits in attentional control and implicit regulatory ability. This interpretation is consistent with recent evidence that the therapeutic effects of anxiety treatment are mediated by early changes in attentional bias (Abend & Bar-Haim 2013), and from behavioral studies suggesting that attentional control improves with anxiety treatment (Pishyar et al., 2008). Additionally, the current findings parallel recent research using a dot-probe task with children suggesting that anxious youth who exhibit pre-treatment attention biases toward threatening stimuli show greater reductions not only in anxiety symptom severity, but also in the likelihood of meeting diagnostic criteria for anxiety disorders following treatment (Waters, Mogg, & Bradley, 2012). These findings provide preliminary evidence at the neural level that youth who recruit less ACC during attempts to direct their attention away from emotional stimuli may benefit the most from anxiety treatment, potentially due to having more to gain in treatment. Future studies are needed to determine whether activation of ACC during the processing of neutral information in the context of emotional distractors improves pre-to-post treatment among youth with anxiety disorders.
Notably, we previously demonstrated in adults that greater dorsal ACC during implicit emotion processing was related to greater anxiety symptom reduction following CBT (Klumpp et al., 2014). One possible explanation for the discrepant findings is that the current sample focused on response to both CBT and SSRI treatment, whereas the previous adult study focused solely on response to CBT. Importantly, previous studies suggest that distinct patterns of ACC activation (i.e., hypo versus hyper) may predict response to CBT versus medication in depression (Ball, Stein, & Paulus, 2013); thus, it is possible that current findings are primarily driven by those treated with SSRI and our analyses may be underpowered to detect differences between the two treatments. Alternatively, there may be differences in the direction of ACC activation depending on age and development. For example, recent work suggests that anxiety is characterized by altered developmental patterns of amygdala connectivity with ACC from childhood into young adulthood (Kujawa, Wu et al., in press). In addition, a separate study shows that age moderates the link between anxiety and the error-related negativity, an event-related potential component that is generated in the ACC (Meyer, Weinberg, Klein, & Hajcak, 2013). Among older children, a larger ERN is significantly related to increased anxiety; however, the relationship between ERN and anxiety appears to be opposite among younger children. Thus, it will be important for future, larger studies to examine how developmental differences impact the relation between ACC activation during emotion processing and treatment response.
Decreased dorsomedial PFC activation during implicit emotion processing also predicted a significant reduction in anxiety symptoms pre-to-post treatment. The dorsomedial PFC is part of an extended medial prefrontal network involved in assessing the value and valence of stimuli and generating adaptive responses that guide behavior (Etkin, Egner, & Kalisch, 2011; Price & Drevets, 2010). Thus, decreased recruitment of this specific region in response to disengaging from emotional information suggests that some individuals may have been more likely to improve if they had a reduced capacity to regulate their responses when attempting to direct their attention away from the emotional stimuli prior to beginning treatment.
The current study extends previous work on pediatric anxiety treatment response by examining the influence of implicit emotion processing. The two previous studies utilizing fMRI to examine predictors of anxiety treatment outcome focused solely on explicit emotion processing, and found that greater amygdala (McClure et al., 2007) and dorsolateral and ventrolateral PFC (Kujawa et al., in press) activation in response to viewing threatening faces predicted better treatment response among anxious youth. In the present study, exploratory post-hoc analyses revealed that treatment response was significantly related to ACC activation during implicit, but not explicit, fear processing. However, the baseline contrast used in these analyses reflects average activation across the entire task, which involves explicit emotion processing. Therefore, future research would benefit from including a separate neutral condition (e.g. “match houses”) in the EFSAT task to more definitively test whether ACC activation is specific to explicit versus implicit processing in relation to pediatric anxiety treatment response.
Results from the current study also showed that the majority of the current sample (79.1%) showed a 35% or greater reduction in PARS severity, which has been associated with treatment response (Caporino et al., 2013). This is noteworthy as this response rate is higher than rates reported in large randomized controlled trials of CBT and SSRI in anxious youth (e.g., Walkup et al., 2008). This is likely due to the current study only including youth who completed treatment, the fMRI scan, and had pre- and post-treatment clinical measures. Future research is needed to evaluate whether neural measures may be useful in predicting treatment engagement and tolerability in children and adolescents with anxiety disorders.
The current findings should be interpreted in the context of several limitations. First, the small sample size may have limited our ability to detect moderators (i.e., age, sex, treatment type) of treatment response. Second, the study sample is heterogeneous with regard to primary diagnoses; although primary diagnoses of SAD and GAD did not moderate the effects, future studies with larger sample sizes are needed to determine if differences are specific to certain anxiety diagnostic groups. Third, the exploratory analytical approach used in the current study to examine emotion-specific effects was circular (i.e., non-independent; for a review, see Kriegeskorte et al., 2010), as the extracted beta weights for the SFG cluster were derived from the Match Shape > Match Face contrast. Therefore, due to potential noise bias, we cannot definitively conclude that the current findings were driven by fearful face distractors. Finally, the study was completed across two sites. Although our findings did not reveal a significant effect of site as a moderator, nonspecific factors from each site, such as patient preference versus random assignment, may have contributed to the findings.
Conclusion
This is the first study to examine the neural correlates of implicit emotion processing in relation to pediatric anxiety treatment response. The findings suggest that less recruitment of SFG, including the dorsal ACC and dorsomedial PFC, during implicit emotion processing predicts a greater reduction in anxiety symptoms pre-to-post treatment. Youth who exhibit reduced activation in these areas while matching shapes in the context of emotional face distractors may have more to gain from CBT and SSRI treatment due to preexisting deficits in attentional control. These findings suggest neuroimaging may be a useful tool for predicting which youth are most likely to benefit from anxiety treatment.
Key points.
Approximately 40-45% of youth with anxiety disorders do not achieve remission following treatment, highlighting the need to identify predictors of treatment response.
The current study examined the neural correlates of directing attention toward and away from emotional faces in relation to pediatric anxiety treatment response.
Less recruitment of superior frontal gyrus, including the dorsal anterior cingulate cortex, during implicit emotion processing predicted a greater reduction in anxiety symptoms pre-to-post treatment.
Youth who exhibit reduced activation in these areas while matching shapes in the context of emotional face distractors may have more to gain from CBT and SSRI treatment due to preexisting deficits in attentional control.
Neuroimaging may be a useful tool for predicting which youth are most likely to benefit from anxiety treatment.
Acknowledgements
This work was supported by National Institute of Mental Health Grant R01-MH086517 to C.S.M. and K.L.P. K.L.B. and A.K. are supported by National Institute of Mental Health Grant T32-MH067631 (Training in the Neuroscience of Mental Health; PI: Mark Rasenick). The authors declare that they have no competing or potential conflicts of interest to declare. They thank James Swain, Gregory Hanna, Elizabeth Koschmann, David Simpson, and Sucheta Connolly, for their help in providing therapeutic services for the current study.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- Abend R, Bar-Haim Y. Threat-related attention bias in the early stages of cognitive–behavior therapy action for panic disorder. Biological Psychiatry. 2013;73(11):1041–1042. doi: 10.1016/j.biopsych.2013.03.016. [DOI] [PubMed] [Google Scholar]
- Ball TM, Stein MB, Paulus MP. Toward the application of functional neuroimaging to individualized treatment for anxiety and depression. Depression and Anxiety. 2014;31(11):920–933. doi: 10.1002/da.22299. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Ernst M. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66(3):275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends in Cognitive Sciences. 2007;11(7):307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7(2):184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox; Presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2–6 June 2002; 2002. Available on CD-ROM in NeuroImage, Vol 16, No 2. [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in cognitive sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Caporino NE, Brodman DM, Kendall PC, Albano AM, Sherrill J, Piacentini J, Rynn M. Defining treatment response and remission in child anxiety: signal detection analysis using the pediatric anxiety rating scale. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(1):57–67. doi: 10.1016/j.jaac.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. John Wiley & Sons Inc; Hoboken, NJ, US: 1999. [Google Scholar]
- Compton SN, Walkup JT, Albano AM, Piacentini JC, Birmaher B, Sherrill JT, Iyengar S. Child/adolescent anxiety multimodal study (CAMS): Rationale, design, and methods. Child and Adolescent Psychiatry and Mental Health. 2010;4(1):1–15. doi: 10.1186/1753-2000-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Ernst M. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65(11):1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kendall PC, Choudhury S, Hudson J, Webb A. The C.A.T. Project Workbook. Worbook Publishing; Ardmore, PA, USA: 2002. [Google Scholar]
- Kendall PC, Hedtke K. Coping Cat Workbook. 2nd edn. Worbook Publishing; Ardmore, PA, USA: 2006. [Google Scholar]
- Klumpp H, Angstadt M, Phan KL. Shifting the focus of attention modulates amygdala and anterior cingulate cortex reactivity to emotional faces. Neuroscience Letters. 2012;514(2):210–213. doi: 10.1016/j.neulet.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Ho SS, Taylor SF, Phan KL, Abelson JL, Liberzon I. Trait anxiety modulates anterior cingulate activation to threat interference. Depression and Anxiety. 2011;28(3):194–201. doi: 10.1002/da.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Fitzgerald DA, Angstadt M, Post D, Phan KL. Neural response during attentional control and emotion processing predicts improvement after cognitive behavioral therapy in generalized social anxiety disorder. Psychological Medicine. 2014;44(14):3109–3121. doi: 10.1017/S0033291714000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Post D, Angstadt M, Fitzgerald DA, Phan KL. Anterior cingulate cortex and insula response during indirect and direct processing of emotional faces in generalized social anxiety disorder. Biology of Mood & Anxiety Disorders. 2013;3(1):1. doi: 10.1186/2045-5380-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Lindquist MA, Nichols TE, Poldrack RA, Vul E. Everything you never wanted to know about circular analysis, but were afraid to ask. Journal of Cerebral Blood Flow & Metabolism. 2010;30(9):1551–1557. doi: 10.1038/jcbfm.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, Phan KL. Enhanced neural reactivity to threatening faces in anxious youth: evidence from event-related potentials. Journal of Abnormal Child Psychology. 2015;43(8):1493–1501. doi: 10.1007/s10802-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Swain JE, Hanna GL, Koschmann E, Simpson D, Connolly S, Phan KL. Prefrontal Reactivity to Social Signals of Threat as a Predictor of Treatment Response in Anxious Youth. Neuropsychopharmacology. doi: 10.1038/npp.2015.368. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Wu M, Klumpp H, Pine DS, Swain JE, Fitzgerald KD, Phan KL. Altered development of amygdala-anterior cingulate cortex connectivity in anxious youth and young adults. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. doi: 10.1016/j.bpsc.2016.01.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Pine DS. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2007;191(1):97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Developmental Cognitive Neuroscience. 2012;1:152–161. doi: 10.1016/j.dcn.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohatt J, Bennett SM, Walkup JT. Treatment of separation, generalized, and social anxiety disorders in youths. American Journal of Psychiatry. 2014;171:741–748. doi: 10.1176/appi.ajp.2014.13101337. [DOI] [PubMed] [Google Scholar]
- Mychailyszyn MP, Méndez JL, Kendall PC. School functioning in youth with and without anxiety disorders: Comparisons by diagnosis and comorbidity. School Psychology Review. 2010;39(1):106–121. [Google Scholar]
- Pine DS. Research review: a neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pishyar R, Harris LM, Menzies RG. Responsiveness of measures of attentional bias to clinical change in social phobia. Cognition and Emotion. 2008;22(7):1209–1227. [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research Units of Pediatric Psychopharmacology Anxiety Study Group The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Phan KL, Angstadt M, Klumpp H, Fitzgerald KD, Monk CS. Altered activation of the rostral anterior cingulate cortex in the context of emotional face distractors in children and adolescents with anxiety disorders. Depression and Anxiety. 2014;31(10):870–879. doi: 10.1002/da.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Iyengar S. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. New England Journal of Medicine. 2008;359(26):2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Mogg K, Bradley BP. Direction of threat attention bias predicts treatment outcome in anxious children receiving cognitive-behavioural therapy. Behaviour Research and Therapy. 2012;50(6):428–434. doi: 10.1016/j.brat.2012.03.006. [DOI] [PubMed] [Google Scholar]