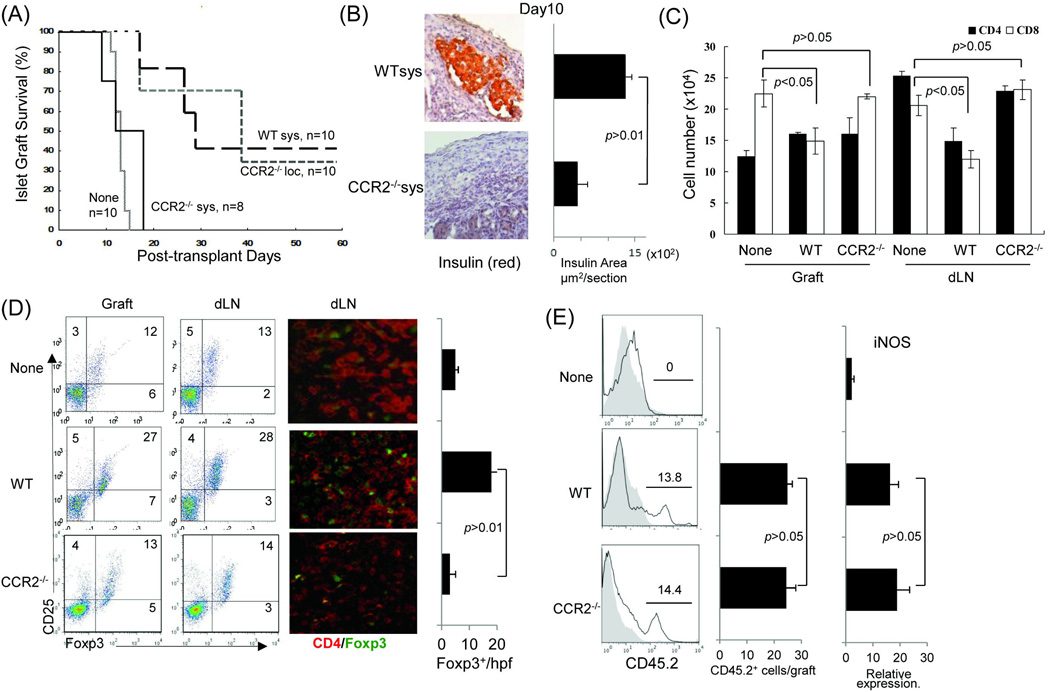
Figure 2. Systemically administered CCR2−/− MDSC lose ability to prolong survival of islet allografts.
Immediately after transplantation of 300 islets (BALB/c) under renal capsule of B6 diabetic STZ induced) recipient, 2 × 106 WT or CCR2−/− MDSC (B6) were intravenously injected (sys). For comparison purpose in a separate group, CCR2−/− MDSC were locally delivered (loc) by being mixed with islets, and then transplanted, as previously described (5). Islet transplantation alone (without MDSC treatment) served as control (None). For mechanistic studies, the recipients treated with systemic administration of WT or CCR2−/− MDSC were sacrificed on POD 10. The islet grafts and draining lymph node were harvested for sections and isolation of cells. (A) Survival of islet allografts. Systemic administration of WT MDSC or local treatment of CCR2−/− MDSC markedly prolonged survival of islet allografts (p<0.05, WT Sys or CCR2−/− Loc vs. None). Systemic administration of CCR2−/− MDSC failed to prolong islet allograft survival (p>0.05, CCR2−/− vs. none; p<0.05, CCR2−/− Sys vs. CCR2−/− Loc). (B) Islet allografts sections were stained with anti-insulin mAb (red). The pictures (left panels) show the presence of functional islets in the recipients receiving systemic administration of WT MDSC, but not in an animal (CCR2−/− MDSC group) with rejected islet grafts. Right panel shows the quantitative data for insulin areas analysis (n=10 in each group). The data were expressed as mean µm2/section ± SD. (C) Poor protection of islet allograft by systemic administration of CCR2−/− MDSC is associated with increased CD8+ T cells. Lymphocytes isolated from islet allografts and draining lymph node (dLN) from the islet allograft recipients receiving systemic administration of WT or CCR2−/− MDSC were stained with anti-CD4, -CD8 mAbs. Lymphocytes isolated from naïve animals served as the controls (None). CD4+ and CD8+ cell number was calculated based on flow analysis, and expressed as mean cell number ± SD (n=3). CD8+ cells, WT sys vs. CCDR2−/− sys, p<0.05 for two-way t test with Bonferroni correction. (D) Systemic administration of CCR2−/− MDSC is not associated with enhanced Treg cell activity. Treg cell activity was examined by flow cytometry for expression of CD25 and Foxp3 gated on CD4+ cells (left panels) or by immunohistochemistry where the cell suspensions were stained with anti-CD4 (red) and -Foxp3 (green) mAbs using fluorescent immunochemical protocol and examined by a microscope. The Foxp3+ cells were counted and expressed as mean Foxp3+ cells/high power field ± SD (n=3). p<0.05 for 2-way t test with Bonferroni correction. (E) Null of CCR2 does not affect MDSC stability in vivo. In a separate experiment, 2 × 106 MDSC propagated from WT (B6) or CCR2−/− mice (both CD45.2+) were mixed with allogeneic islets (BALB/c), and transplanted under the renal capsule of congenic B6 recipients (CD45.1+) (n=3). Islet allograft transplantation alone served as controls (None). On POD7, the grafts were peeled off under a microscope for leukocyte isolation. Cells were double stained with anti-CD11b and -CD45.2, analyzed by flow cytmetry gated on CD11b+ cells, and displayed as hystograms. The number is percentage of CD45.2+ cells (left panels). The absolute number of CD45.2+ cells were calculated based on the flow analysis (middle panel). The myeloid cells were purified using CD11b+ beads for isolation of mRNA. Expression of iNOS was determined by qPCR (right panel). The data were analyzed by t test (two-tailed) with Bonferroni correction.