Figure 1. GSK3 phosphorylates Olig2 at S10.
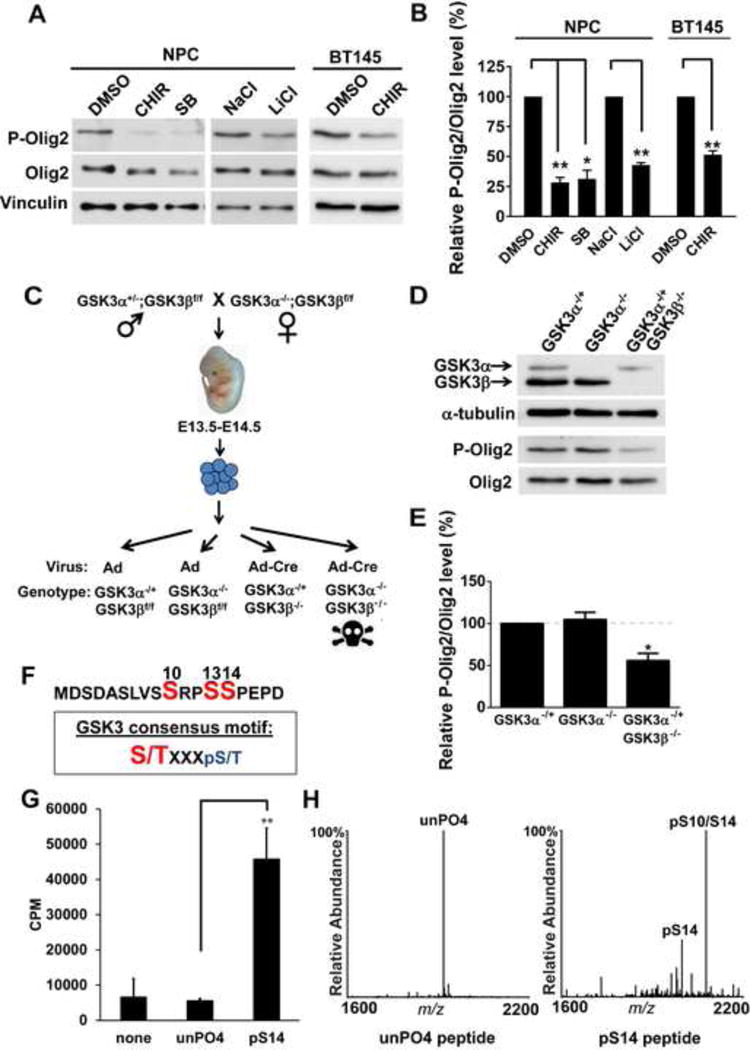
(A and B) Inhibition of GSK3 results in reduced Olig2 phosphorylation. Mouse NPCs and BT145 human glioma cells treated with GSK3 inhibitors, CHIR99021(CHIR), SB216763(SB) or LiCl for 4 hours show a decrease in P-Olig2 level, in comparison to control cells that were treated with either DMSO or NaCl. 5 μm CHIR99021, 10 μm SB216763, 10 mM of NaCl and 10 mM LiCl were used. Relative P-Olig2/Olig2 levels were quantified and compared between control and inhibitor-treated group. Data were analyzed by t-test and are represented as mean ± SEM. *p<0.05, **p<0.01, n=3. (C) A schematic diagram shows generation of mouse GSK3α- and β-knockout NPC lines. Control adeno or adeno-cre virus was introduced to generate GSK3α- or GSK3β-knockout NPC lines. (D and E) GSK3α and β function redundantly to phosphorylate Olig2. P-Olig2 levels were examined in GSK3α−/+, GSK3α −/−, and GSK3α−/+GSK3β−/− NPCs. Relative P-Olig2/Olig2 were quantified and compared between different groups. Data were analyzed by t-test and are represented as mean ± SEM. *p<0.05, n=3. (F) GSK3 consensus motif fits the S10 site upon phosphorylation at S14. S/T (highlighted with red), the kinase’s target Serine/Threonine residue; X, any amino acid; pS, phosphorylated serine. (G) in vitro kinase assay shows that GSK3 phosphorylates Olig2 N-terminal peptide, however, it requires a priming phosphorylation at S14. Synthetic Olig2 N-terminal peptides (a.a.1–18) were used, and both unphosphorylated Olig2 N-terminal peptide (unPO4) and phosphorylated peptide at S14 (pS14) were tested. Reactions without peptides (none) served as negative control. Data were analyzed by t-test and are represented as mean ± SEM. n=3; **p<0.01. (H) Mapping the GSK3 phosphorylation sites by mass spectrometry analysis. in vitro kinase reactions were analyzed by MALDI-MS and −MS/MS. The doubly phosphorylated peptide at S14 and S10 is indicated by pS14/S10.
