Figure 4. Phosphorylation of the triple serine motif enables formation of a hexa-phosphoserine acid blob in the amino terminus of Olig2.
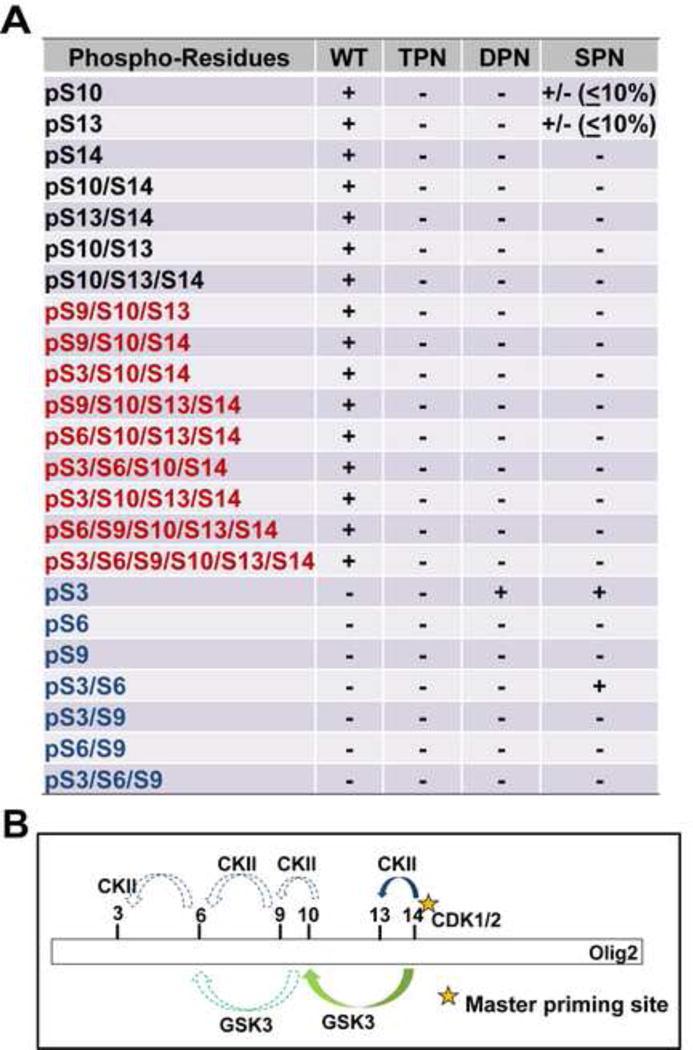
(A) Summary of mass spectrometric analyses on Olig2 N-terminal phosphorylated peptides detected in Olig2-null NPCs that were transduced with WT, TPN (S10A/S13A/S14G), DPN (S13A/S14G) or SPN (S14G) Olig2. Black font: phosphorylated peptides identified in previous study (Sun et al., 2011); Red font: newly identified phosphorylated peptides in WT-Olig2 sample; Blue font: contingent phospho peptides that are undetectable or barely detectable in WT-Olig2 sample. +, present; +/− detected but with low level; −, undetectable. (B) A sequentially priming phosphorylation cascade. Schematic diagram shows how the triple serine motif creates priming sites for additional phosphorylations at S3, S6 and S9 that create a hexaphosphate acid blob in the Olig2 amino terminus.
