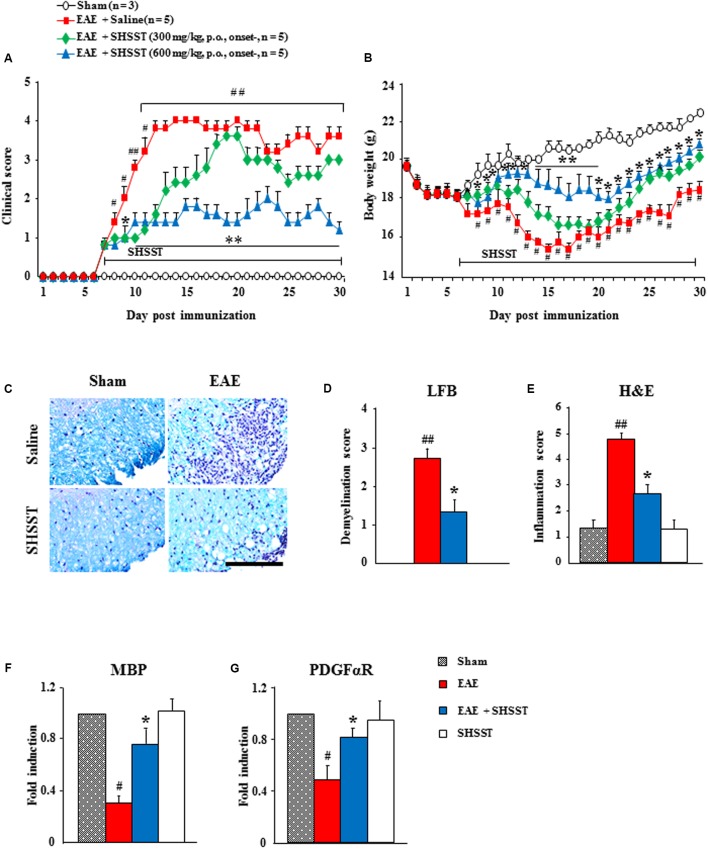
FIGURE 3.
The effect of onset-treatment with SHSST on clinical symptoms and demyelination of EAE mice. (A,B) Following immunization, the clinical signs of mice from sham, EAE, EAE + SHSST, and SHSST groups were scored daily (A) and weighed daily (B), until 30 days had passed. SHSST (300 or 600 mg/kg) was orally administrated daily, beginning from the onset stage (day 6–8 after immunization) of clinical signs of EAE. (C–E) Lumbar spinal cord sections were obtained from each group at day 14–16 post-immunization. The sections were stained with luxol fast blue and counterstained by hematoxylin (C); and the levels of demyelination (D) and inflammation (E) were quantified. (F,G) Lysate of the lumbar spinal cord, obtained from each group at day 14–16 post-immunization, was analyzed for mRNA expression of MBP (F) and PDGFαR (G) by real-time PCR. Bar = 10 μm. Each of the quantified data are expressed as mean clinical scores, body weight, demyelination score, infiltration score, or fold induction ±SEM. (Student t-tests were performed for A and B; and ANOVA testing was performed; #p < 0.05 and ##p < 0.01 versus the sham group; ∗p < 0.05 and ∗∗p < 0.01 versus the EAE group.)