Introduction
Immune checkpoint inhibitors are able to harness and stimulate the immune system's innate antitumor capabilities. One of these targeted checkpoints is the programmed cell death-1 (PD-1) pathway. When PD-1 is bound to its ligands, it transduces a coinhibitory signal to activated T cells allowing for immune system dampening and peripheral tolerance. Cancer cells exploit this pathway by expressing their own PD-1 ligands enabling them to evade immune system recognition and elimination.1, 2 Nivolumab is a PD-1 antibody that disrupts this T-cell inhibitory pathway. It is approved by the US Food and Drug Administration for the treatment of metastatic melanoma, non–small cell lung cancer, renal cell carcinoma, and classical Hodgkin lymphoma.1 Immune-related adverse events (irAEs), such as colitis, endocrinopathies, and dermatitis, have been well documented in patients treated with checkpoint inhibitors.3 We report a case of immune-related cutaneous sarcoidosis in a patient with lung adenocarcinoma on nivolumab monotherapy.
Case report
A 63-year-old African-American woman had stage IV epidermal growth factor receptor mutation-negative lung adenocarcinoma diagnosed 2 years before presentation. The patient was started on 4 cycles of carboplatin (area under the curve 6), 900 mg, paclitaxel, 200 mg/m2, and bevacizumab, 15 mg/kg every 3 weeks, followed by bevacizumab, 15 mg/kg maintenance therapy every 3 weeks. Fourteen months later, imaging showed an increase in pleural metastatic disease, and the patient was started on nivolumab, 3 mg/kg every 2 weeks.
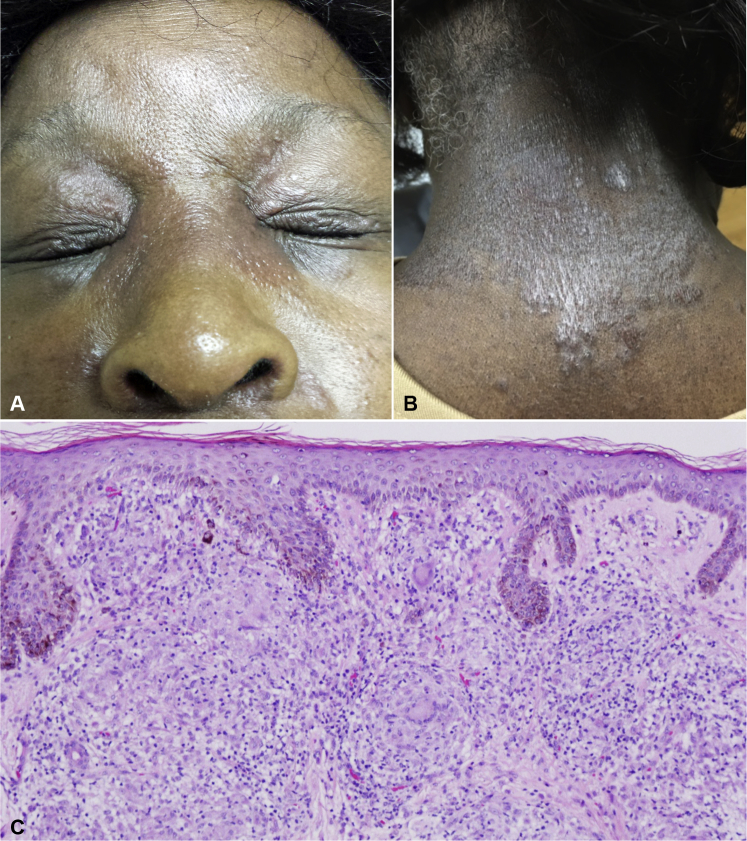
After 7 cycles of nivolumab, the patient presented to the dermatology department with a 3-week history of a severely pruritic waxing and waning cutaneous eruption on the periorbital skin and posterior neck. On examination, the patient had around fifteen 2- to 4-mm scaly, erythematous papules coalescing into plaques on the periorbital areas bilaterally (Fig 1). There were also around twenty 4- to 6-mm scaly, erythematous papules coalescing into plaques on the posterior neck (Fig 1). The patient was prescribed fluocinonide 0.05% ointment to the neck twice daily and hydrocortisone 2.5% ointment to the face twice daily for suspected contact dermatitis. On follow-up 2 weeks later, the patient did not improve, and the lesions had progressed to red-brown plaques now involving the glabella and nasal bridge. Given the lack of improvement and progression, a punch biopsy of the neck lesion was performed, which found nodular collections of epithelioid and multinucleated histiocytes surrounded by a sparse lymphocytic infiltrate throughout the dermis (Fig 1). Fite and periodic acid–Schiff–diastase staining were negative for atypical mycobacterial and fungal infection. Considering the patient's nivolumab history, the diagnosis was therapy-related cutaneous sarcoidosis. The patient's nivolumab infusion was held for 1 cycle, and she was started on methylprednisolone, 24 mg for 1 day, and tapered down to 4 mg/d over the next 6 days. The lesions initially regressed after 48 hours but flared up around 48 hours after completing the steroid taper. Although some research has found that systemic corticosteroids do not seem to diminish the efficacy of immunotherapy, the patient was treated with low-dose prednisone, 10 mg once daily. However, the patient only showed complete resolution after hydroxychloroquine, 200 mg twice daily, was added. She was able to continue her nivolumab therapy and has shown stable disease on a follow-up positron emission tomography scan 1 month later.
Fig 1.
A, Firm, erythematous papules and plaques on the glabella and periorbital area with spread to the nasal bridge. B, Scaly papules and plaques on the posterior neck. C, Nodular collections of epithelioid and multinucleated histiocytes surrounded by a sparse infiltrate of lymphocytes are present throughout the dermis. (Hematoxylin-eosin stain; original magnification: ×10.)
Discussion
To date, several cases of immune checkpoint-induced sarcoidosis have been reported, most occurring in melanoma patients treated with ipilimumab, a cytotoxic T-lymphocyte–associated protein 4 (CTLA-4) inhibitor.2 Two cases involved nivolumab as a monotherapy, both of which were in patients with melanoma.2, 4 Another case reported nivolumab-related sarcoidosis in a lung cancer patient, but the patient was on ipilimumab and nivolumab combination therapy.3 In this case, suppressing both PD-1 and CTLA-4 obscured the pathway responsible for the observed irAE. Our case of immune-related cutaneous sarcoidosis in a lung cancer patient on nivolumab monotherapy further supports the theory that PD-1 blockade may contribute to the pathogenesis of immune-related sarcoidosis independently from CTLA-4 blockade.
The PD-1 pathway and its role in immunity are exceedingly complex. Once activated, T cells, B cells, macrophages, and dendritic cells all express the PD-1 receptor. The corresponding PD-1 ligands (PD-L1 and PD-L2) can be induced on many cells, particularly on antigen-presenting cells.5 The PD-1–PD-L1 interaction inhibits T-cell proliferation and the production of key inflammatory cytokines such as interferon-γ, tumor necrosis factor-α, and interleukin-2.5 PD-1 also contributes to immunologic peripheral tolerance by aiding in the conversion of T cells into regulatory T cells.6 In addition to its role in the PD-1 pathway, PD-L1 serves as an analog to CTLA-4, allowing it to participate in a second, parallel immunoinhibitory pathway.7
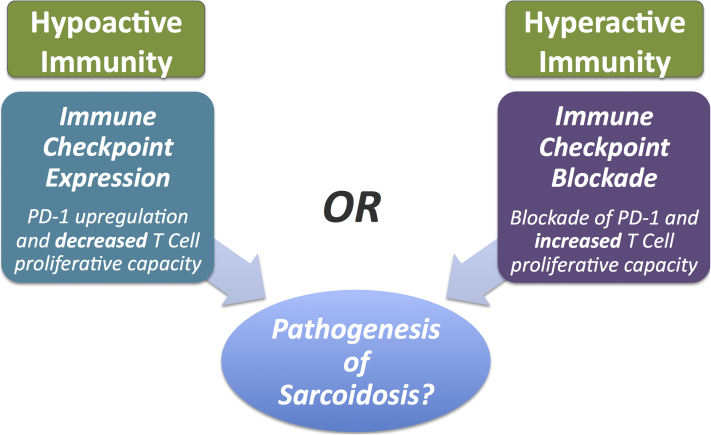
In our patient, nivolumab blocked PD-1's immune suppression leading to sustained or increased immunogencity. However, CTLA-4 and PD-L1 were unaffected and free to continue causing T-cell inhibition via the CTLA-4 pathway. This PD-1 inhibition, in the absence of CTLA-4 inhibition, provides some insight into the pathogenesis of sarcoidosis. However, an overly simplistic paradigm may be inaccurate because there is also paradoxical evidence of PD-1's role in sarcoidosis. A recent study found that PD-1 was upregulated on CD4+ T cells in sarcoidosis patients compared with healthy controls.8 The authors posit that PD-1 overexpression may decrease T-cell proliferative capacities leading to the immunologic derangements associated with sarcoidosis. This finding is counter to what our case exhibits with PD-1 blockade appearing to be involved in the pathogenesis of sarcoidosis (Fig 2). The PD-1 upregulation, however, may be a secondary response rather than the initiating event in the case of sarcoidosis; thus, more research is needed.
Fig 2.
The pathogenesis of sarcoidosis and competing paradigms.
The salient clinical correlate to therapy-related sarcoidosis is the concept of tumor pseudoprogression and its impact on patient management. Although our patient did not present with pulmonary sarcoidosis, clinicians must be cautious not to mistake a granulomatous irAE, which can be highlighted on positron emission tomography/computed tomography scan, for progression of a treated cancer or metasteses.9 This is important for all malignancies but especially critical in the treatment of lung cancer. Most therapy-related sarcoidosis cases respond to topical or systemic steroids and regress.4 It is encouraging that our patient's sarcoidosis is improving on hydroxychloroquine, as it is nonimmunosuppressive and has even been theorized to have anticancer effects by inhibiting autophagy, a cellular defense mechanism against metabolic stress.10
Conclusion
Clinicians must continue to be aware of potential irAEs as immune checkpoint inhibitors remain a part of our oncology armament. Although the clear mechanisms on how PD-1 is related to sarcoidosis remain elusive, its general participation in the disease process becomes more evident as new cases of PD-1 inhibitor-related sarcoidosis are reported.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Opdivo (nivolumab) Prescribing Information, May 2016. Food and Drug Association Website. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125554s019lbl.pdf. (Updated May 10, 2016. Accessed September 12, 2016).
- 2.Danlos F.X., Pagès C., Baroudjian B. Nivolumab-induced sarcoid-like granulomatous reaction in a patient with advanced melanoma. Chest. 2016;149(5):e133–e136. doi: 10.1016/j.chest.2015.10.082. [DOI] [PubMed] [Google Scholar]
- 3.Suozzi K.C., Stahl M., Ko C.J. Immune-related sarcoidosis observed in combination ipilimumab and nivolumab therapy. JAAD Case Rep. 2016;2(3):264–268. doi: 10.1016/j.jdcr.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montaudié H., Pradelli J., Passeron T., Lacour J.-P., Leroy S. Pulmonary sarcoid-like granulomatosis induced by nivolumab [e-pub ahead of print] Br J Dermatol. 2016 doi: 10.1111/bjd.14808. [DOI] [PubMed] [Google Scholar]
- 5.Keir M.E., Liang S.C., Guleria L. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francisco L.M., Salinas V.H., Brown K.E. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butte M.J., Keir M.E., Phamduy T.B., Freeman G.J., Sharpe A.H. PD-L1 interacts specifically with B7-1 to inhibit T cell proliferation. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celada L.J., Rotsinger J.E., Young A. Programmed death-1 inhibition of PI3K/AKT/mTOR signaling impairs sarcoidosis CD4+ T cell proliferation. Am J Respir Cell Mol Biol. 2017;56:74–82. doi: 10.1165/rcmb.2016-0037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shetty A., Carter J.D. Sarcoidosis mimicking lymphoma on FDG-PET imaging. Radiology Case Reports. 2011;6(2):409. doi: 10.2484/rcr.v6i2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaravadi R.K., Lippincott-Schwartz J., Yin X.-M. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17(4):654–666. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]