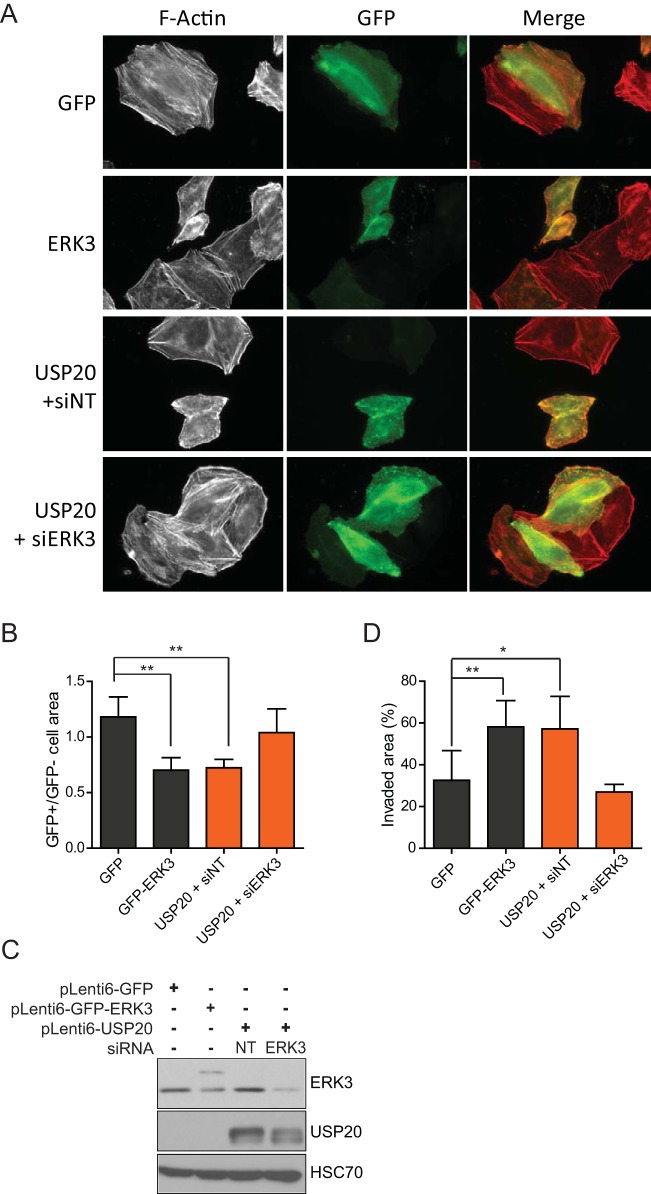
FIG 8.
USP20 regulates actin cytoskeleton dynamics and migration via ERK3. (A) HeLa cells were transfected with the indicated constructs in the absence or presence of SMARTpool ERK3 siRNAs. After 48 h, the cells were fixed, and actin filaments were stained with rhodamine-conjugated phalloidin. Actin cytoskeleton organization was visualized by epifluorescence microscopy. (B) The cellular adhesive area was quantified and is presented as a ratio of transfected (GFP-positive [GFP+])/untransfected (GFP-negative [GFP−]) cells. A minimum of 50 cells were counted, and the bar graph represents the means ± standard deviations of data from at least three independent experiments. (C and D) HeLa cells were infected with lentiviral vectors encoding GFP-ERK3 or USP20, and populations of transduced cells were selected with blasticidin. USP20-overexpressing cells were transfected with nontarget or SMARTpool ERK3 siRNAs. After 24 h, the cell lysates were analyzed for ERK3 expression by immunoblotting (C) and cells were plated at 90% confluence (D). Twenty-four hours after seeding, the confluent monolayer of cells was scraped with a sterile P200 tip. Cell migration was assessed by measuring the surface of the wound area 24 h later by phase-contrast microscopy. The bar graph represents the means ± standard deviations of data from at least three independent experiments. Statistical significance was determined by an unpaired t test. **, P < 0.01; *, P < 0.05.