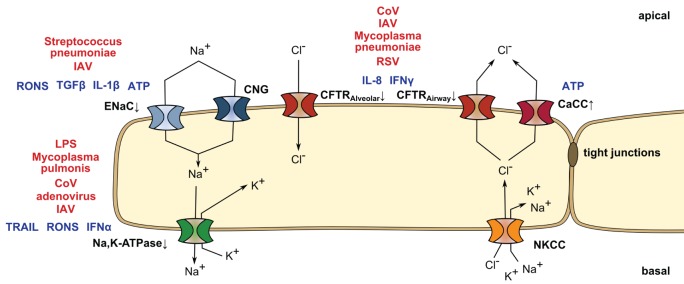
Figure 1.
Mediators released in pulmonary infection and their effects on ion homeostasis. Ion transport of the lung epithelial cell is mediated by various ion channels and pumps. Sodium enters the epithelial cell via the apical cyclic nucleotide-gated cation channel (CNG) or the epithelial sodium channel (ENaC), that can be downregulated by reactive oxygen and nitrogen species (RONS) and ATP, transforming growth factor beta (TGF-β) or interleukin-1 beta (IL-1β) upon Streptococcus pneumoniae and influenza A virus (IAV) infection. Sodium is secreted at the basolateral side by the Na,K-ATPase (NKA), which is modulated in lipopolysaccharide (LPS)-induced lung injury as well as upon Mycoplasma pulmonis, IAV, coronavirus (CoV), or adenovirus challenge. RONS, interferon-alpha (IFN-α), and TNF-related apoptosis-inducing ligand (TRAIL) lead to a decrease in NKA abundance or activity. In parallel, chloride is taken up (alveolar epithelium) or secreted (airway) by the cystic fibrosis membrane conductance regulator (CFTR) and secreted by apical Ca2+-activated ion channels (CaCC), supported by basolateral potassium channels (not shown) and Na+/K+/2Cl− cotransporters (NKCC). While extracellular ATP enhances chloride secretion by CaCC, CFTR action is reduced by IFN-γ and interleukin-8 (IL-8) in CoV, IAV, respiratory syncytial virus (RSV), or Mycoplasma pneumoniae infection.