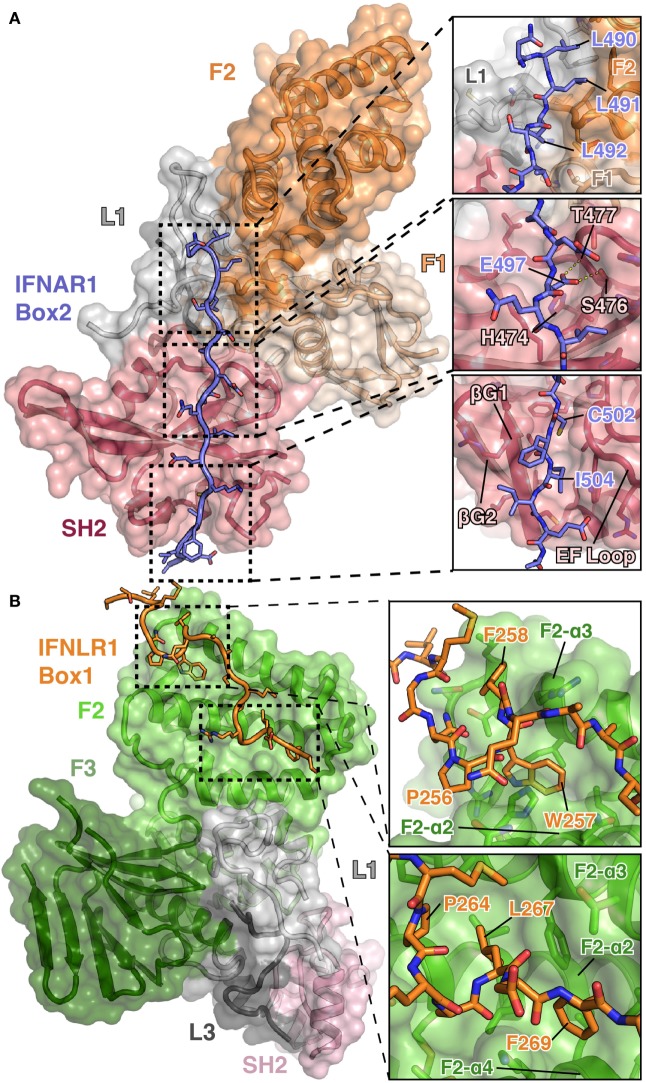
Figure 3.
Detailed views of the interactions between TYK2–IFNAR1 Box2 and JAK1 IFNLR1 Box1. (A) Overview of the interaction between TYK2 FERM and SH2 (colored as in Figures 1A,B) domains in complex with the IFNAR1 Box2 region (left, PDB ID 4PO6). Detailed interactions are shown for three regions of the TYK2–IFNAR1 complex (right panels). In the N-terminal tri-leucine region, Leu490, Leu491, and Leu492 of IFNAR1 pack against a hydrophobic groove between the TYK2 F1 (tan), L1 (gray), and F2 (orange) domains (top panel). In the center of the interaction, Glu497 of IFNAR1 forms hydrogen bonds with Ser476 and Thr477 (middle panel). At the C-terminus of IFNAR1, Cys502 and Ile504 insert into a groove between the β-G1/2 hairpin and EF loop of the TYK2 SH2 domain (bottom panel). (B) Overview of the bipartite interaction between JAK1 FERM and SH2 (colored as in Figure 1C) domains in complex with the IFNLR1 Box1 region (left, 5IXD). Detailed interactions are shown for two regions of the JAK1–IFNLR1 complex (right panels). At the first site, IFNLR1 residue Trp257 inserts itself into a groove between JAK1 F2-α2 and F2-α3 (top panel). At the second site, hydrophobic residues Pro264, Leu267, and Phe269 of IFNLR1 pack into a groove formed by JAK1 F2-α2, F2-α3, and F2-α4 (bottom panel).