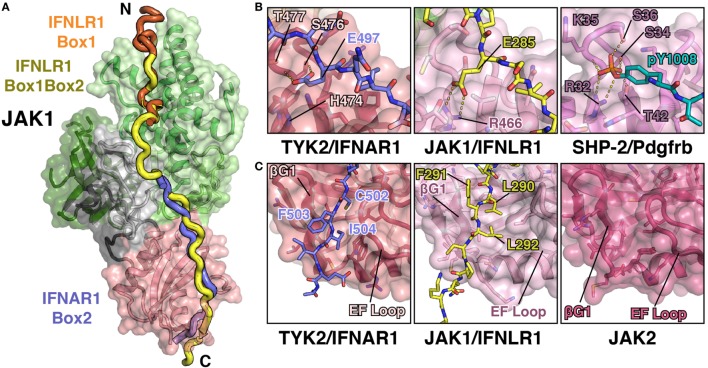
Figure 5.
JAK1 and TYK2 recognize the Box2 motif via a conserved mechanism. (A) Cartoon representation of JAK1 (colored as in Figure 1C) bound to IFNLR1 receptor peptide (yellow) containing both the Box1 and Box2 motifs (PDB ID 5L04). For comparison, the IFNLR1 Box1 motif (orange) and the IFNAR1 Box2 (blue) are shown after superposition of JAK1 and TYK2, respectively. (B) Detailed comparison of the pTyr/Glu binding pocket of the TYK2 (PDB ID 4PO6), JAK1, and SHP-2 (PDB ID 1AYA) SH2 domains. TYK2 (left) contains a histidine residue (H474) at the base of the pocket, while JAK1 and SHP-2 have an arginine at this position that forms a salt bridge with either the glutamate in IFNLR1 or phosphate group in the PDGF receptor peptide. (C) Detailed comparison of the Box2 binding groove of TYK2, JAK1, and JAK2. IFNAR1 residues Cys502 and Ile504 pack into a groove in the TYK2 SH2 domain between the β-G1/2 hairpin and EF loop (left). In the JAK1/IFNLR1 structure, Leu290 and Leu292 pack into this groove (center). The SH2 domain of JAK2 (PDB ID 4Z32) maintains this conformation in the absence of receptor (right).