The 2013–2016 Ebola epidemic in West Africa was larger than all previous Ebola outbreaks combined and was unique in the breadth of its geographical dispersion. The size and scope of this epidemic provided the potential to achieve significant advances in understanding the disease and to improve outbreak prevention strategies and public health responses for the future. This special issue gathers together research on key aspects of the disease to ensure that lessons can be learned and improvements made for the benefit of future responses. It is the first compendium that addresses the largest Ebola epidemic in history.
The nature of research into neglected tropical diseases, particularly those which only result in occasional outbreaks, is that publication is infrequent. This special issue builds on a comprehensive previous special issue published following the second largest outbreak to date in Kikwit, Democratic Republic of Congo in 1995. This was published in Journal of Infectious Diseases (1999) and contains the key body of scientific work underpinning much of what we knew about Ebola prior to the 2013–2016 epidemic [1].
This special issue reflects the multidisciplinary approach required to deal with a global health crisis and offers new research to enhance the biological, epidemiological, clinical and operational Ebola knowledge-base. Owing to the unprecedented scale of cross-national virus transmission, we also include dedicated research on the behavioural and socio-political factors that both drove and eventually played a part in controlling the epidemic. For example, Wilkinson et al. [2] provide detailed accounts of the landscape of local outbreak responses which complements work by Jalloh et al. [3]: a ‘Knowledges, Attitudes and Practices’ survey conducted through the epidemic in Sierra Leone and Guinea, the first such study related to Ebola. Additionally, Wenham et al. [4] and Ross [5] detail the high-level architecture of outbreak response management.
The most important step forward during the epidemic was the development of a safe and highly effective vaccine and this publication includes the first review of the vaccine candidates [6]. In addition, the use of mathematical modelling and computationally intensive statistical techniques has become a mainstay of outbreak control analysis and this work, which has not previously been applied during Ebola outbreaks, is reflected in the current issue: e.g. Funk et al. [7] and Mbala et al. [8]. Whitty [9] highlights the crucial role of integrating such diverse disciplines to optimize the outbreak response and he hypothesizes that without this the epidemic would have been significantly worse.
Nevertheless, a key opportunity was missed: this epidemic provided the potential to collect enough data over a sufficient period of time to develop, test and implement therapeutic, prophylactic and non-pharmaceutical control strategies for Ebola. Previous outbreaks had been over too quickly with too few cases to set up rigorously controlled studies. Therefore, this epidemic provided a rare opportunity. However, notwithstanding the significant contribution to future disease control of the effective rVSV ZEBOV vaccine that underwent successful phase III clinical trials in Guinea [10], insufficient data were gathered to develop clinical knowledge regarding the basic supportive management and appropriate therapeutic protocols for Ebola patients. This missed opportunity is exemplified by the editors' inability to commission a clinical research article for this special issue. While many factors influencing disease outcome remain elusive, we have included a number of papers focused on clinical aspects of the outbreak: Rojek et al. [11] review the developments in pharmaceutical therapeutics that occurred during the epidemic and suggest barriers to regulatory approval; Garske et al. [12] conduct comprehensive analyses of line list data to evaluate predictors of case fatality risk; and Logue et al. [13] provide a case study of Ebola diagnostics and training in field laboratories during the outbreak.
Unfortunately, the sparsity of data collected also serves to limit our understanding of the effectiveness of different interventions. This in turn restricts our ability to better understand the answers to key public health questions such as: which transmission interventions work best?; and in which order should they be deployed? It is only through indirect and retrospective analysis—e.g. Funk et al. [6], Skrip et al. [13] and Senga et al. [14]—that we can start to address these questions given there were no direct observational studies conducted during the epidemic. The ineffective implementation of interventions, together with the lack of data, not only hampered research efforts but also had a direct effect on the spread of the epidemic. Senga et al. estimate that only a small fraction of new cases reported over the first six months were known contacts of prior cases (documented on contact tracing lists) meaning that contact tracing in the initial stages of the epidemic was highly ineffective. This led to unmonitored, sustained transmission that, in all probability, prevented the epidemic from being contained.
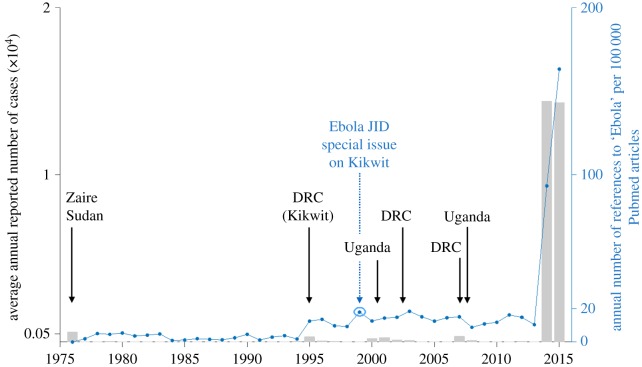
The paucity of data sits in stark contrast to the surge in publications about Ebola that appeared during the first year of the crisis as the epidemic ran uncontrolled (figure 1). Cori et al. [14] provide a comprehensive description of the data and data management techniques we will need to control future outbreaks, with an important discussion of the need for rapid transfer of useably formatted data to facilitate real-time analysis of outbreak control. While resolving this issue was not feasible during the 1995 Kikwit outbreak, technological developments by the 2010s meant this should not have remained a problem.
Figure 1.
Number of Ebola virus cases reported by year (bars) and number of published articles containing the word ‘Ebola’ by year (points). Labels (black) correspond to previous outbreaks where more than 100 cases were reported. Previous special issue documenting the outbreak in Kikwit in the Democratic Republic of the Congo (DRC, formerly Zaire) in blue. (Online version in colour.)
Over 20 years after publication of the Kikwit Ebola special issue, woefully little progress has been made in controlling and treating Ebola. As Coltart et al. [15] highlight, many of the same concerns and conclusions documented in the 1990s by Heymann et al. pursuant to the Kikwit outbreak remain relevant now and match the recommendations made by the expert panels reviewing the recent epidemic. This is reinforced by Piot et al. [16], who detail examples of recommendations common to both the 1995 and 2013–2016 outbreaks. These include the need for stronger infectious disease surveillance (both national and global), improved international preparedness to provide support when similar outbreaks occur, more broad-based international health regulations, and continued and coordinated Ebola research (diagnostics, patient management and identification of the natural reservoir).
The impact of this epidemic on individuals, healthcare, societies and economies was profound and will last many years beyond the end of the epidemic. Had the lessons of previous outbreaks been heeded by the global community, thousands of deaths could have been prevented. Looking forward, we sincerely hope that recommendations will be enacted and will translate into tangible solutions. Although Ebola outbreaks remain inevitable, these recommendations can and should prevent future outbreaks from reaching the same size and scale as the devastating 2013–2016 epidemic.
Acknowledgements
We wish to thank the following people who acted as referees on the papers within this issue: Christian Althaus, Gregory Atkins, Steve Bellan, Neil Bentley, Catherine Bolten, David Brett-Major, Colin Brown, James Childs, Dan Cooper, Henry Dowlen, Rosalind Eggo, Marshall Elliott, Mosoka Fallah, Nigel Field, Judith Glynn, Mike Jacobs, Philip Krause, Dominic la Hausse de Lalouviere, Adam Levine, Fred Martineau, Amanda McClelland, Laura Miller, Harriet Mills, Kate Mitchell, Oliver Morgan, Martial Ndeffo Mbah, Geraldine O'Hara, Gustaf Rydevik, Laura Skrip, Conall Watson, Clare Wenham, Jimmy Whitworth, Annie Wilkinson and Mark Woolhouse.
Biographies
Guest Editor biographies
 Katherine Atkins received her BSc in mathematics from the University of Edinburgh, her MRes from the University of York in applied mathematics and her PhD from the University of Edinburgh in Biological Sciences. After postgraduate studies, she conducted postdoctoral work at the Yale School of Public Health. She is currently Assistant Professor of Infectious Disease Modelling at the London School of Hygiene and Tropical Medicine. Her research focuses on mathematical modelling on infectious diseases, with a particular emphasis on vaccine-preventable diseases.
Katherine Atkins received her BSc in mathematics from the University of Edinburgh, her MRes from the University of York in applied mathematics and her PhD from the University of Edinburgh in Biological Sciences. After postgraduate studies, she conducted postdoctoral work at the Yale School of Public Health. She is currently Assistant Professor of Infectious Disease Modelling at the London School of Hygiene and Tropical Medicine. Her research focuses on mathematical modelling on infectious diseases, with a particular emphasis on vaccine-preventable diseases.
 John Edmunds is Professor of Infectious Disease Modelling at the London School of Hygiene and Tropical Medicine, and Dean of the Faculty of Epidemiology and Population Health. John's research focuses on modelling the spread of infectious diseases and the design of efficient control programmes. He has been involved in helping the UK Government to plan and prepare for pandemic flu and similar emergencies over a number of years. He has been a member of a number of national and international advisory committees, including WHO's Ebola Science Committee, the UK's New and Emerging Respiratory Virus Technical Advisory Group (NERVTAG) and various subcommittees of the Joint Committee on Vaccines and Immunisation (JCVI).
John Edmunds is Professor of Infectious Disease Modelling at the London School of Hygiene and Tropical Medicine, and Dean of the Faculty of Epidemiology and Population Health. John's research focuses on modelling the spread of infectious diseases and the design of efficient control programmes. He has been involved in helping the UK Government to plan and prepare for pandemic flu and similar emergencies over a number of years. He has been a member of a number of national and international advisory committees, including WHO's Ebola Science Committee, the UK's New and Emerging Respiratory Virus Technical Advisory Group (NERVTAG) and various subcommittees of the Joint Committee on Vaccines and Immunisation (JCVI).
 Cordelia Coltart is a clinical academic in Infectious Diseases at UCL. She graduated in Medicine from Imperial College, London and gained an MPH from Harvard University. She has also obtained a Bachelor's degree in Human Genetics from UCL, a DTM&H from LSHTM, and a certificate in Humanitarian Studies from Harvard, MIT and Tufts. She has worked across a wide range of settings, both nationally and internationally, including clinical work in the UK and sub-Saharan Africa, medical relief work in post-earthquake Haiti, public health experience at the WHO in Geneva, and in UK health policy during a secondment to the Chief Medical Officer's Clinical Advisors Scheme. Most recently, she worked in Sierra Leone during the Ebola outbreak. She is currently undertaking a PhD, funded by the Wellcome Trust, investigating how molecular epidemiology can enhance traditional epidemiology with reference to transmission dynamics during epidemics.
Cordelia Coltart is a clinical academic in Infectious Diseases at UCL. She graduated in Medicine from Imperial College, London and gained an MPH from Harvard University. She has also obtained a Bachelor's degree in Human Genetics from UCL, a DTM&H from LSHTM, and a certificate in Humanitarian Studies from Harvard, MIT and Tufts. She has worked across a wide range of settings, both nationally and internationally, including clinical work in the UK and sub-Saharan Africa, medical relief work in post-earthquake Haiti, public health experience at the WHO in Geneva, and in UK health policy during a secondment to the Chief Medical Officer's Clinical Advisors Scheme. Most recently, she worked in Sierra Leone during the Ebola outbreak. She is currently undertaking a PhD, funded by the Wellcome Trust, investigating how molecular epidemiology can enhance traditional epidemiology with reference to transmission dynamics during epidemics.
Competing interests
We declare we have no competing interests.
Funding
C.E.M.C. is supported by the Wellcome Trust (grant code: 106551/Z/14/A). K.E.A. was funded by the National Institute of Health Research Health Protection Research Unit (NIHR HPRU) in Immunisation at the London School of Hygiene and Tropical Medicine in partnership with Public Health England.
References
- 1.Peters CJ, LeDuc JW (eds). 1999. Ebola: the virus and the disease. J. Infect. Dis. 179(suppl 1). [DOI] [PubMed] [Google Scholar]
- 2.Wilkinson A, Parker M, Martineau F, Leach M. 2017. Engaging ‘communities’: anthropological insights from the West African Ebola epidemic. Phil. Trans. R. Soc. B 372, 20160305 ( 10.1098/rstb.2016.0305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalloh MF, et al. 2017. Assessments of Ebola knowledge, attitudes and practices in Forécariah, Guinea and Kambia, Sierra Leone, July–August 2015. Phil. Trans. R. Soc. B 372, 20160304 ( 10.1098/rstb.2016.0304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wenham C. 2017. What we have learnt about the World Health Organization from the Ebola outbreak. Phil. Trans. R. Soc. B 372, 20160307 ( 10.1098/rstb.2016.0307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross E. 2017. Command and control of Sierra Leone's Ebola outbreak response: evolution of the response architecture. Phil. Trans. R. Soc. B 372, 20160306 ( 10.1098/rstb.2016.0306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambe T, Bowyer G, Ewer KJ. 2017. A review of phase I trials of Ebola virus vaccines: what can we learn from the race to develop novel vaccines? Phil. Trans. R. Soc. B 372, 20160295 ( 10.1098/rstb.2016.0295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funk S, et al. 2017. The impact of control strategies and behavioural changes on the elimination of Ebola from Lofa County, Liberia. Phil. Trans. R. Soc. B 372, 20160302 ( 10.1098/rstb.2016.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mbala P, Baguelin M, Ngay I, Rosello A, Mulembakani P, Demiris N, Edmunds WJ, Muyembe J-J. 2017. Evaluating the frequency of asymptomatic Ebola virus infection. Phil. Trans. R. Soc. B 372, 20160303 ( 10.1098/rstb.2016.0303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitty CJM. 2017. The contribution of biological, mathematical, clinical, engineering and social sciences to combatting the West African Ebola epidemic. Phil. Trans. R. Soc. B 372, 20160293 ( 10.1098/rstb.2016.0293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henao-Restrepo AM, et al. 2015. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 386, 857–866. ( 10.1016/S0140-6736(15)61117-5) [DOI] [PubMed] [Google Scholar]
- 11.Rojek AM, Horby PW. 2017. Offering patients more: how the West Africa Ebola outbreak can shape innovation in therapeutic research for emerging and epidemic infections. Phil. Trans. R. Soc. B 372, 20160294 ( 10.1098/rstb.2016.0294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garske T, et al. 2017. Heterogeneities in the case fatality ratio in the West African Ebola outbreak 2013–2016. Phil. Trans. R. Soc. B 372, 20160308 ( 10.1098/rstb.2016.0308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logue CH, et al. 2017. Case study: design and implementation of training for scientists deploying to Ebola diagnostic field laboratories in Sierra Leone: October 2014 to February 2016. Phil. Trans. R. Soc. B 372, 20160299 ( 10.1098/rstb.2016.0299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cori A, et al. 2017. Key data for outbreak evaluation: building on the Ebola experience. Phil. Trans. R. Soc. B 372, 20160371 ( 10.1098/rstb.2016.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coltart CEM, Lindsey B, Ghinai I, Johnson AM, Heymann DL. 2017. The Ebola outbreak, 2013–2016: old lessons for new epidemics. Phil. Trans. R. Soc. B 372, 20160297 ( 10.1098/rstb.2016.0297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piot P, Coltart CEM, Atkins KE. 2017. Preface: ‘The 2013–2016 Ebola epidemic: data, decision-making and disease control’. Phil. Trans. R. Soc. B 372, 20170020 ( 10.1098/rstb.2017.0020) [DOI] [PMC free article] [PubMed] [Google Scholar]