ABSTRACT
The periodontal ligament (PDL), which connects the teeth to the alveolar bone, is essential for periodontal tissue homeostasis. Although the significance of the PDL is recognized, molecular mechanisms underlying PDL function are not well known. We report that mohawk homeobox (Mkx), a tendon-specific transcription factor, regulates PDL homeostasis by preventing its degeneration. Mkx is expressed in the mouse PDL at the age of 10 weeks and expression remained at similar levels at 12 months. In Mkx−/− mice, age-dependent expansion of the PDL at the maxillary first molar (M1) furcation area was observed. Transmission electron microscopy (TEM) revealed that Mkx−/− mice presented collagen fibril degeneration in PDL with age, while the collagen fibril diameter gradually increased in Mkx+/+ mice. PDL cells lost their shape in Mkx−/− mice, suggesting changes in PDL properties. Microarray and quantitative polymerase chain reaction (qPCR) analyses of Mkx−/− PDL revealed an increase in osteogenic gene expression and no change in PDL- and inflammatory-related gene expression. Additionally, COL1A1 and COL1A2 were upregulated in Mkx-overexpressing human PDL fibroblasts, whereas osteogenic genes were downregulated. Our results indicate that Mkx prevents PDL degeneration by regulating osteogenesis.
KEY WORDS: Mohawk, PDL homeostasis, Collagen, Osteogenic change, Mouse
Summary: Analyses of knockout mice indicate that the transcription factor mohawk regulates osteogenic changes associated with age and is essential for homeostasis of the periodontal ligament.
INTRODUCTION
The periodontal ligament (PDL), one of the periodontal tissues, connects the teeth with the alveolar bone. The PDL plays diverse roles, supplying nutrients to periodontal tissues and providing sensory input to the masticatory system, and has a self-repair ability. Thus, the PDL is important for maintaining homeostasis of periodontal tissues, including the gingival tissue, dental pulp and teeth (Avery, 2000; Beertsen et al., 1997). Once a tooth erupts, the PDL receives occlusal loading. The loss of PDL due to inflammation, injury or aging leads to ankylosis, which causes many clinical problems, such as root resorption. Although the PDL is composed of heterogeneous cell types such as fibroblasts, osteoblasts, osteoclasts, cementoblasts, endothelial cells, sensory cells and their progenitor or stem cells (Beertsen et al., 1997; Maeda et al., 2011; Ueda-Maeda, 2006), fibroblasts are responsible for the fibrous attachment of the PDL. The components of PDL include type I collagen (70-80%), other types of collagen (type III, IV, V, VI and XII collagens), and other extracellular matrix proteins such as periostin (Postn), glycoproteins and proteoglycans, all of which are mainly produced by PDL fibroblasts (Aukkarasongsup et al., 2013; Beertsen et al., 1997; Ma et al., 2011; Rios et al., 2005; Sloan, 1979; Ahuja, 2012).
In recent years, the genes underlying PDL function have been extensively studied. For example, scleraxis (Scx) is known to be an important transcriptional factor for PDL development through expression of type I collagen and Postn (Hasegawa et al., 2015; Seo et al., 2004; Takimoto et al., 2015; Yokoi et al., 2007). Mutations in type I collagen and Postn cause the abnormal development of PDL in mice (Rios et al., 2005). In contrast, the molecular mechanism responsible for the maintenance of PDL homeostasis has never been clarified.
We previously developed a database termed EMBRYS (http://embrys.jp/embrys/html/MainMenu.html), representing the expression data of 1520 transcription factors during mouse embryo development. Based on this database, we identified transcription-regulating genes expressed during murine molar development (Uchibe et al., 2012). The transcription factor mohawk homeobox (Mkx), a member of the three-amino-acid loop extension (TALE) superclass of atypical homeobox genes, is expressed in the embryonic progenitor cell populations of the cartilage, skeletal muscle, tendons and bones, in addition to the tips of the ureteric buds in the metanephric kidneys and the testis cords of the male gonad testis in the mouse embryo (Anderson et al., 2006). Additionally, Mkx plays a crucial role in tendon maturation by regulating the expression of type I collagen (Ito et al., 2010; Liu et al., 2010; Nakamichi et al., 2016; Onizuka et al., 2014; Suzuki et al., 2016). However, the significance of Mkx in the PDL has never been explored.
Herein, we report that Mkx is expressed in the PDL and regulates collagen fibril degeneration with age. Importantly, artificial control of Mkx expression in human PDL fibroblasts led to the successful management of the quality of PDL fibroblasts. Thus, our results indicate that Mkx plays a unique and important role in PDL homeostasis and provide a novel insight into the biology of the PDL.
RESULTS
Mkx expression in the PDL
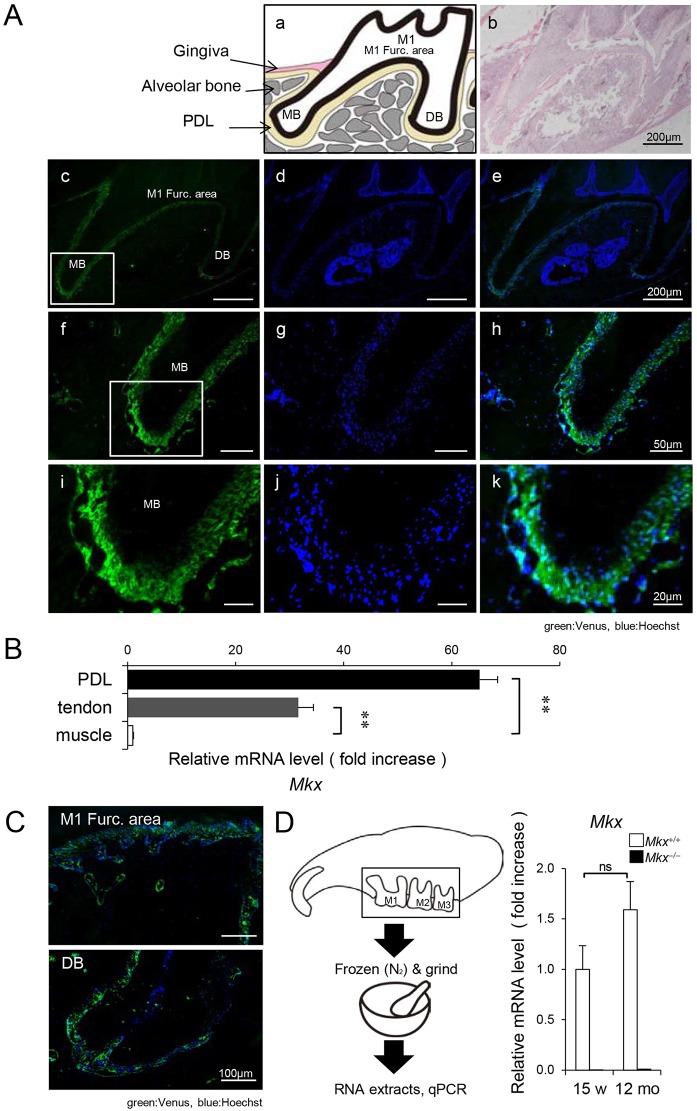
To establish the role of Mkx in periodontal tissues, we analyzed Mkx expression in 10-week-old Mkx+/− mice, which express Venus fluorescent protein under the control of the Mkx promoter. Venus signals were located in the PDL region between the teeth and the alveolar bone, but Venus was not detected in the pulp, dentin or enamel tissue (Fig. 1A). qPCR analysis revealed that the level of Mkx mRNA in the PDL tissue of 10-week-old Mkx+/+ mice was much higher than that in the tendon, in which Mkx is known to be expressed (Fig. 1B) (Ito et al., 2010; Liu et al., 2010). These observations indicate that Mkx is specifically and highly expressed in the PDL. Moreover, we verified whether Mkx is also expressed at later stages. In 12-month-old mice, Venus signals remained similar to those observed in 10-week-old mice (Fig. 1C). Using qPCR analysis, we confirmed mRNA expression of the Mkx gene in the maxillary molar region, which includes the PDL, in 12-month-old Mkx+/+ mice (Fig. 1D).
Fig. 1.
Expression of the transcription factor mohawk homeobox in periodontal ligaments. (A) Venus signals in the maxillary first molar (M1) region. (a) Schematic representation of M1, MB (mesial-buccal) and DB (distal-buccal) roots. (b) H&E staining of the M1 region. Venus signals (c,f,i) and Hoechst staining (d,g,j) were merged (e,h,k). Scale bars: 200 µm (b-e), 50 µm (f-h) and 20 µm (i-k), respectively. (B) Analysis of Mkx expression in the PDL by qPCR. Tendon, positive control; muscle, negative control (n=6 each; mean±s.e.m.; **P<0.01). (C) Merged Venus signals and Hoechst staining in the M1 furcation area and DB root region of 12-month-old mice. Scale bars: 100 µm. (D) Analysis of Mkx expression in the maxillary molar region around M1-M3, also including alveolar bone and PDL. Maxillary molar regions were extracted and frozen with liquid nitrogen and ground (n=3 each; mean±s.e.m.; ns, not significant).
Acceleration of PDL degeneration in Mkx−/− mice
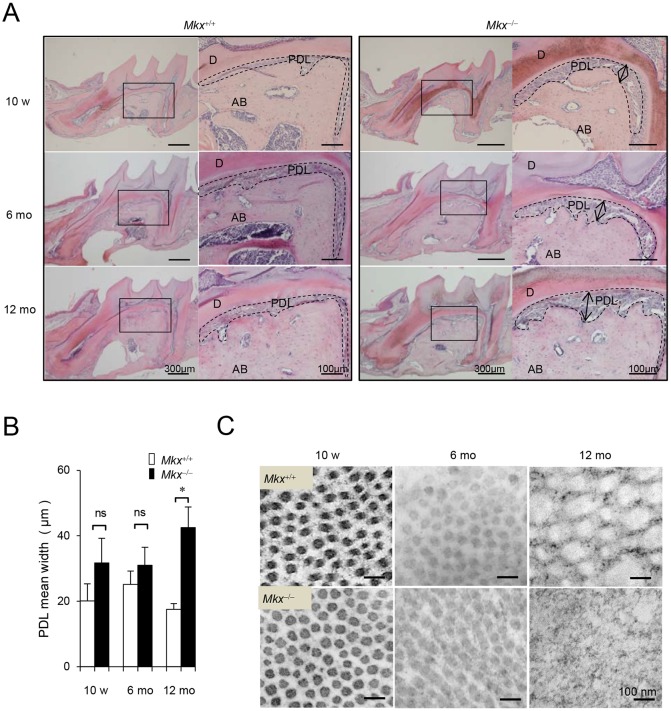
The observation of restricted expression of Mkx in the periodontal tissue led us to investigate its role in the PDL. Although no significant changes were observed in the PDL of 10- or 6-month-old Mkx−/− mice compared with Mkx+/+, the PDL space of the 1st molar (M1) furcation area expanded, and the alveolar bone surface became uneven and jagged in 12-month-old Mkx−/− mice (Fig. 2A, Figs S1 and S2). In contrast, expansion of the PDL space was not observed in the area around the medial, distal-root or each apical area of the 1st molar in 12-month-old Mkx−/− mice (Fig. 2A, Fig. S2). The expansion was also not observed in the PDL of 2nd or 3rd molars (Fig. S3). However, the PDL space in the M1 furcation area clearly expanded, with a 1.5- to 2.4-fold increase in 12-month-old Mkx−/− mice compared with Mkx+/+ mice (Fig. 2B). In addition, TEM analysis revealed that the diameter of PDL collagen fibrils in Mkx+/+ mice increased gradually over 12 months and their cross-sectional shape remained nearly round. However, in Mkx−/− mice, the maintenance mechanism of collagen fibrils completely collapsed by 12 months, as indicated by the small diameter and irregularity of the margin of collagen fibrils in cross-sectional images (Fig. 2C). Thus, collagen fibrils in the PDL degenerated with aging. These results suggest that Mkx plays an important role in the homeostasis of PDL tissues, but not in PDL development. However, we observed that thickening of PDL collagen fibrils with age in Mkx+/+ mice has little effect on the physical strength of PDL collagen. We hypothesize that this thickening is one of the degenerative changes in PDL.
Fig. 2.
Age-related defects of the PDL in Mkx-deficient mice. (A) H&E staining of the M1 region in 10-week-, 6-month- and 12-month-old Mkx+/+ or Mkx−/− mice. D, dentin; AB, alveolar bone; PDL, periodontal ligament. Arrows indicate expansion of the PDL space. (B) Quantitative analysis for width of the PDL space in Mkx+/+ or Mkx−/− mice (n=5, 6 and 6 for 10-week-, 6-month- and 12-month-old Mkx+/+; n=3, 6, and 10 for 10-week-, 6-month- and 12-month-old Mkx−/− mice, respectively; mean±s.e.m.; ns, not significant; *P<0.05). (C) TEM analysis of collagen fibrils in the M1 furcation area. Images are representative examples.
Osteogenic changes of the PDL in Mkx−/− mice
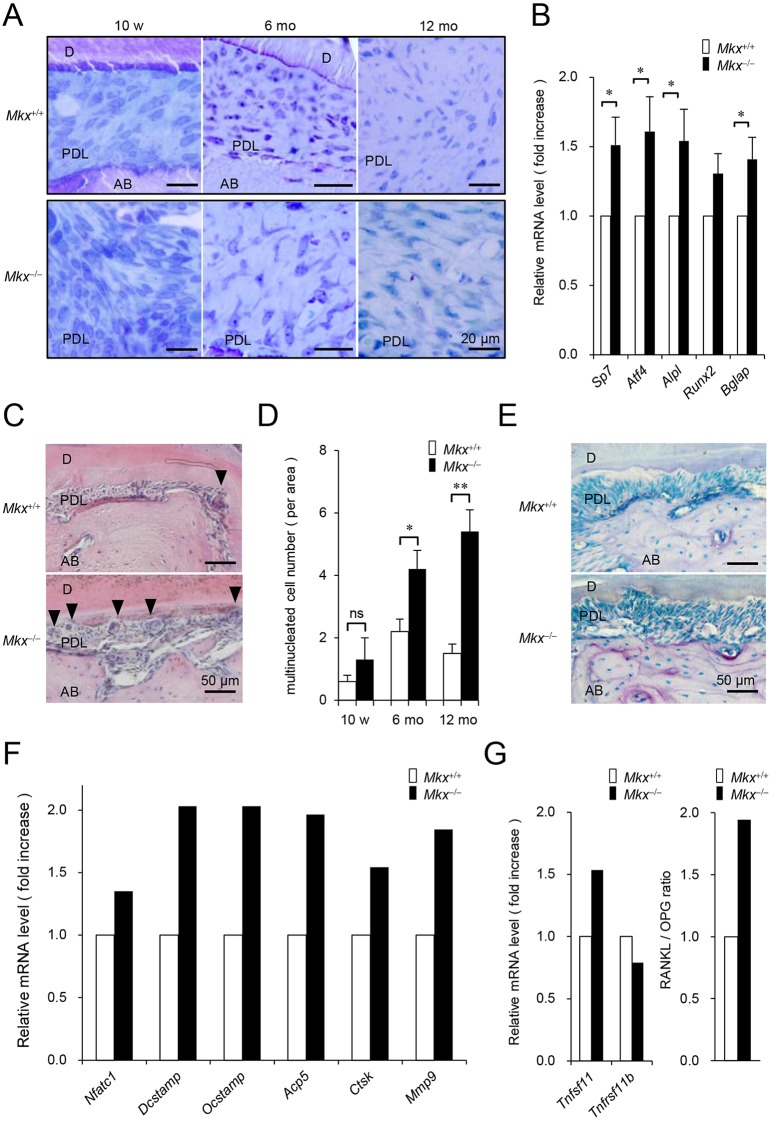
To gain further insights into the role of Mkx in PDL maintenance, the PDL tissues were investigated at the cellular level. In Mkx+/+ mice, cells with various shapes were observed, supporting a previous report that the PDL tissue is composed of a variety of cells in addition to PDL fibroblasts (Beertsen et al., 1997). In contrast, although the cells in Mkx−/− mice were normal at the age of 10 weeks, the cells in 6-month- and 12-month-old Mkx−/− mice were uniformly large. In addition, cells with a spindle shape, which is a typical feature of fibroblasts, were not observed in 12-month-old Mkx−/− mice (Fig. 3A). Moreover, TEM analysis using higher magnification (Fig. S4) indicated that PDL cells in 10-week-old Mkx+/+ and Mkx−/− mice had similar shapes. These spindle-shaped cells, each of which had a large ovoid nucleus and well-developed rough endoplasmic reticulum, produced large quantities of collagen. These cells have several morphological characteristics of fibroblasts. Additionally, in Mkx+/+ mice, cells in the PDL had fibroblast-like features and produced large quantities of collagen. However, in 12-month-old Mkx−/− mice, these cells resembled osteoblasts or osteocytes, which are known to have an irregular outline and are covered within a lacuna surrounded by extracellular matrix (osteocyte lacuna). Surprisingly, these cells produce large quantities of black ovoid matrix vesicles, which probably contain hydroxyapatite crystals and are an indication of initial ossification. Taken together with the accumulation of substances such as calcified deposits in the Mkx−/− PDL tissue, these cell properties in Mkx−/− mice suggest changes in PDL cells that are characteristic of osteogenic cells (Fig. S4).
Fig. 3.
Morphologic and characteristic changes of cells in the PDL space and alveolar bone destruction in Mkx-deficient mice. (A) Toluidine Blue staining of M1 furcation area in 10-week-, 6-month- and 12-month-old Mkx+/+ or Mkx−/− mice. AB, alveolar bone; D, dentin; PDL, periodontal ligament. (B) Expression of osteogenic genes in PDL of 10-week-old Mkx+/+ or Mkx−/− mice (n=17 each; mean±s.e.m.; *P<0.05). (C) H&E staining of the M1 furcation area in 12-month-old Mkx+/+ or Mkx−/− mice. Arrowheads indicate multi-nucleated cells with more than two nuclei, counted to avoid including two neighboring cells. (D) The number of multi-nucleated cells in 10-week-, 6-month- and 12-month-old Mkx+/+ or Mkx−/− mice (n=5, 6 and 6 for 10-week-, 6-month- and 12-month-old Mkx+/+; n=3, 6 and 10 for 10-week-, 6-month- and 12-month-old Mkx−/− mice, respectively; mean±s.e.m.; ns, not significant; *P<0.05, **P<0.01). (E) TRAP staining of the M1 furcation area in 12-month-old Mkx+/+ or Mkx−/− mice. (F) Expression of osteoclast-related genes determined by microarray analysis of the PDL from 10-week-old Mkx+/+ or Mkx−/− mice (n=1 each). (G) Expression of Tnfsf11 and Tnfrsf11b determined by microarray analysis of PDL from 10-week-old Mkx+/+ or Mkx−/− mice (left) and RANKL/OPG ratio (right) (n=1 each).
Consistently, the expression of osteoblast-related genes such as Sp7 (encoding osterix), Atf4, Alpl (encoding alkaline phosphatase), Runx2 and Bglap (encoding osteocalcin) was increased in the PDL tissue of Mkx−/− mice (Fig. 3B), whereas the expression of PDL-related genes was almost unchanged (Fig. S5).
Furthermore, we noted that multi-nucleated cells appeared around the site of alveolar bone erosion (Fig. 3C,D). Tartrate-resistant acid phosphatase (TRAP) staining analysis also indicated enhancement of bone resorption in the 12-month-old Mkx−/− mice at the M1 furcation area (Fig. 3E). In addition, since the expression of osteoclast-related genes increased in the PDL tissue of Mkx−/− mice (Fig. 3F), it is possible that the multi-nucleated cells are bone-resorbing osteoclasts. It is well known that osteogenic cell lines such as osteoblasts and osteocytes produce an osteoclast differentiation factor, RANKL (encoded by Tnfsf11), and an osteoclast differentiation inhibitory factor, OPG (encoded by Tnfrsf11b), and that the ratio of RANKL/OPG is crucial for osteoclast differentiation (Aubin and Bonnelye, 2000). As shown in Fig. 3G, the RANKL/OPG ratio was elevated upon the increase of RANKL and decrease of OPG in Mkx−/− mice. Generally, RANKL expression in fibroblasts is induced by the inflammatory cytokines, TNF, IL-1β and IL-6, in certain pathological conditions such as rheumatoid arthritis. However, the expression of inflammatory cytokines was not changed in the PDL tissue of Mkx−/− mice (Fig. S6), suggesting that RANKL induction is independent of inflammation and supporting the cell-autonomous change of PDL cells to osteogenic cells.
Regulation of PDL cell homeostasis by artificial Mkx expression
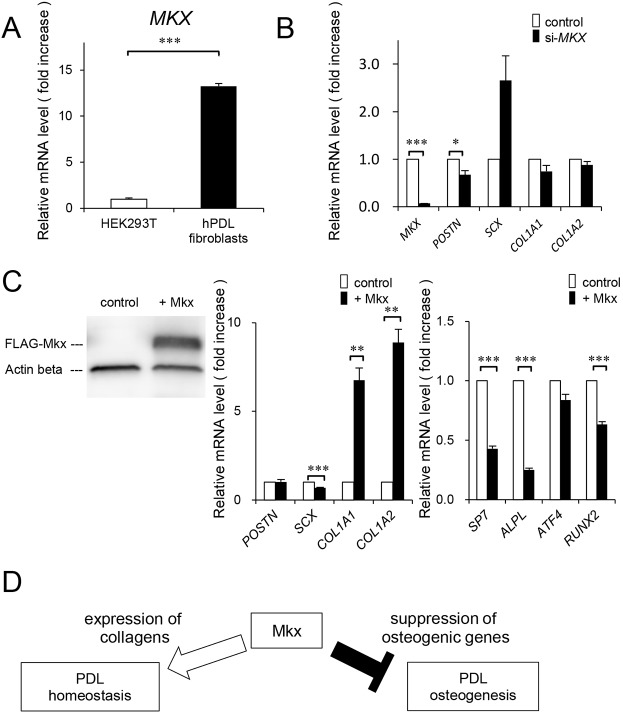
The finding of the significant role of MKX in the maintenance of PDL tissue prompted us to examine the effect of artificial expression of Mkx in PDL cells in vitro. For this purpose, we used human PDL (hPDL) fibroblasts, in which MKX is highly expressed (Fig. 4A). When MKX expression was decreased, there was a slight but significant decrease in POSTN expression. However, expression of SCX, which is known to be an important transcriptional regulator of PDL development (Brent et al., 2003; Seo et al., 2004), tended to increase, but not significantly, and the expression of collagens such as COL1A1 and COL1A2 was not affected (Fig. 4B). These results were consistent with the observation that PDL-related gene expression was almost unchanged in the PDL tissue of Mkx−/− mice (Fig. S5). In contrast, Mkx overexpression in hPDL fibroblasts resulted in a dramatic increase in COL1A1 and COL1A2 expression and a slight decrease in SCX expression. More importantly, the expression of osteogenic genes such as SP7, ALPL and RUNX2 was suppressed by Mkx overexpression in hPDL fibroblasts (Fig. 4C). These results indicate that Mkx regulates PDL homeostasis by supporting the expression of collagens and by inhibiting degeneration through the suppression of osteogenic-related gene expression in PDL cells (Fig. 4D).
Fig. 4.
Effect of MKX knockdown or Mkx overexpression on gene expression patterns of human PDL fibroblasts. (A) Analysis of MKX expression in hPDL fibroblasts at passage 7 (P7) and HEK293T as a negative control (n=3 each). (B) Expression of the PDL-related genes in MKX-knockdown hPDL fibroblasts (n=6 each). (C) Expression of FLAG-Mkx in hPDL fibroblasts determined by western blot analysis (left), expression of PDL-related extracellular matrix proteins (center) (n=6) and osteogenic genes in control or FLAG-Mkx-expressing hPDL fibroblasts (right) (n=6). (D) Schematic diagram of Mkx function in PDL homeostasis. All data are mean±s.e.m.; *P<0.05, **P<0.01, ***P<0.001.
DISCUSSION
PDL plays an essential role in periodontal tissues by stabilizing the teeth and absorbing mechanical stress during the chewing process. Thus, several dental diseases such as chronic periodontitis and periapical periodontitis are closely related to PDL disorders. Although PDL reconstitution has not been established, PDL regeneration should improve tooth substitution techniques (Chen and Jin, 2010; Dangaria et al., 2011). The identification of a key transcription factor regulating PDL development and homeostasis should contribute to therapeutic advancement in the dental field.
Here, we showed that Mkx is specifically expressed in the PDL, but not in the enamel, dentin or dental pulp. This restricted Mkx expression in the PDL suggests that Mkx is involved in PDL functions. The PDL of 10-week-old Mkx−/− mice did not show significant phenotypes, whereas the PDL of the Mkx−/− mice at 6 months and 12 months showed accelerated degenerative changes compared with the PDL of age-matched Mkx+/+ mice, including (1) expansion of the PDL at the furcation area, (2) abnormal collagen fibril structure, (3) loss of PDL cell morphology, (4) disordered gene expression profile, (5) alveolar bone surface irregularity and (6) abnormal appearance of multi-nucleated cells in PDL tissues. We previously showed that the platysma tendons, as well as the other tendons in Mkx−/− mice, are markedly hypoplastic; however, there was no difference in body weight between Mkx+/+ and Mkx−/− mice, suggesting that food intake was not altered in Mkx−/− mice (Ito et al., 2010). These observations, along with the fact that Mkx is highly expressed in PDL (Fig. 1), indicate the direct function of Mkx in PDL homeostasis.
Degenerative changes and PDL space expansion are commonly observed in periapical periodontitis, which is a severe and incurable dental disease. Damaged PDL in periapical periodontitis causes serious symptoms such as percussion pain, local fever, gingival swelling and cyst formation, and may eventually lead to tooth loss. Additionally, it is known that the PDL in periapical periodontitis is excessively expanded with the accumulation of inflammatory cells (Page and Schroeder, 1976). We previously observed that Mkx expression in human tendon cells is downregulated by inflammatory signals such as IL-1β stimulation (Nakahara et al., 2013). Inflammatory markers were not elevated in Mkx−/− mice, suggesting that PDL degeneration is due to changes in the expression of PDL- and osteogenic-related genes rather than inflammation (Fig. S6), or at least indicating that the PDL was not undergoing acute inflammatory changes. Although the pathogenesis of periapical periodontitis is not fully elucidated, chronic inflammation in periapical periodontitis may also promote the PDL degenerative process via Mkx expression.
The molecular mechanisms of the PDL degenerative changes in Mkx−/− mice can be explained by the function of Mkx as a transcription factor. In keeping with the findings of the PDL analysis in Mkx−/− mice, gene expression analysis in MKX-silencing and Mkx-overexpression experiments using hPDL fibroblasts revealed that MKX strongly and specifically promotes the expression of a set of extracellular matrix genes related to PDL tissue formation, including COL1A1 and COL1A2, which are similar to those reported in mesenchymal stem cells (Liu et al., 2015; Otabe et al., 2015). In particular, in PDL cells, Postn, which is among the extracellular matrix adhesion molecules mainly expressed in the bone, periosteum, and PDL tissues (Kruzynska-Frejtag et al., 2004; Ma et al., 2011; Rios et al., 2005), was significantly decreased in the M1 PDL in 10-week-old Mkx−/− mice in vivo. In addition, Postn-deficient mice exhibit a phenotype of vertical PDL expansion and alveolar bone resorption in the M1 furcation area (Horiuchi et al., 1999; Rios et al., 2008), suggesting that PDL phenotype in Mkx−/− mice could, in part, be due to the suppression of Postn. If PDL width is expanded because of the absence of Postn and consequently loses its physical strength, it is possible that PDL has a compensating mechanism, and it changes to an osteogenic phenotype. In addition, we recently reported ChIP-seq analysis and found Mkx binding peaks around the Sp7 and Runx2 gene loci, indicating that these genes are also potential targets of Mkx (Suzuki et al., 2016).
Scx is another transcription factor involved in PDL formation (Takimoto et al., 2015). Notably, we found that Scx expression is regulated by Mkx in PDL cells. It is also interesting to note that Scx expression increased in the absence or suppression of Mkx, suggesting a compensatory mechanism that prevents a drastic decrease in Col1a1, Col1a2 and other PDL-related genes.
In aged mice, smooth alveolar bone surface of PDL gradually changes to a jagged or uneven boundary with age (Severson et al., 1978). However, the surface irregularity was severe in Mkx−/− mice, suggesting that the absence of Mkx gene expression promotes the aging process of PDL. In Mkx−/− PDL, we also observed osteogenic differentiation of PDL cells and increased number of migrated multi-nucleated (osteoclast-like) cells with an upregulation of the RANKL/OPG ratio. These results indicated that Mkx may be crucial for maintaining PDL cell characteristics, and loss of Mkx may cause abnormal differentiation into osteogenic cells. Although we could not exclude the possibility that multiple cell populations including progenitor cells and putative stem cells in PDL may affect the osteogenic changes in Mkx−/− PDL, we did confirm that dysregulation of MKX affects osteogenic genes in hPDL cells. In this regard, alveolar bone resorption in the PDL of Mkx−/− mice could be a secondary effect of these osteogenic changes and decreased stability of PDL.
In humans, the PDL gradually degenerates during the aging process. In elderly subjects, the PDL shows atrophic changes and loss of function (Sloan, 1995). We reported that MKX expression in human tendon cells is reduced with age, indicating a function of MKX in tendon homeostasis (Nakahara et al., 2013). In this regard, the detailed analysis of potential Mkx expression changes with age, both in humans and mice, may lead us to uncover new information about the pathogenesis of PDL-related diseases. The potential application of MKX to promote PDL-related gene expression should provide a basis for advancement in PDL regenerative medicine.
MATERIALS AND METHODS
Experimental animals
Venus knock-in Mkx deletion mice have been previously described (Ito et al., 2010). Briefly, the Venus gene encoding an improved green fluorescent protein (GFP) was inserted in exon 2 of the Mkx gene. Mkx+/+ and Mkx−/− mice (C57BL/6N background) were obtained from an intercross of Mkx+/− mice. All animal experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee at the Tokyo Medical and Dental University (approval no. 0160118A, 2011-198C17).
Histological analysis and immunohistochemistry
For histological analysis, maxillary periodontal tissues of 10-week-, 6-month- and 12-month-old mice were fixed with PBS containing 4% paraformaldehyde (4% PFA/PBS) at 4°C overnight and decalcified with Morse's solution for 4-8 weeks (Morse, 1945). After the tissues were dehydrated, embedded in paraffin and sectioned, each section was stained with hematoxylin and eosin (H&E) (Wako, Saitama, Japan). For immunohistochemistry, anesthetized mice were perfused with 4% PFA/PBS containing 20% sucrose, and periodontal tissues around the maxillary first molar of 10-week-old mice were dissected. The paraffin sections were prepared as described previously (Takimoto et al., 2015). Anti-GFP antibody MBL-598 (Medical & Biological Laboratories, Nagoya, Japan, MBL-598, 1:500) and Alexa Fluor 488-conjugated secondary antibody (Molecular Probes, Thermo Fisher Scientific, Waltham, MA, USA, 1:500) were used for detection of the Venus fluorescent protein. The sections were incubated with the primary and secondary antibodies for 1 h at room temperature and nuclei were stained with Hoechst 33342 (Fisher, 1:2000). After immunohistochemical analysis, the sections were counterstained with H&E (Section Lab, Hiroshima, Japan). These experiments were performed with at least three independent samples to confirm reproducibility.
Measurements of PDL width in the M1 furcation area
PDL width in the M1 furcation area was measured as shown in Fig. S1 using a microscope measurement tool (cellSens, Olympus, Tokyo, Japan). The points were at equal distance from each other and the edge of the maxillary first molar (M1) furcation area. The average value of the two points (points d and e in Fig. S2) was determined.
Transmission electron microscopy (TEM) and Toluidine Blue staining
Tissues, including the maxillary 1st molar and surrounding bone, were dissected and were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB) for 2 h. After washing with 0.1 M PB for 1 h, the tissues were post-fixed with 1% OsO4 in 0.1 M PB for 2 h. The tissues were dehydrated in a graded series of ethanol and embedded in Epon812. Before sectioning, Epon block samples were prepared by cutting the enamel area. Ultrathin (90 nm) sections were collected on copper grids, double-stained with uranyl acetate and lead citrate, and then examined by TEM (H-7100, Hitachi, Tokyo, Japan) (Ichinose et al., 2010). Semi-thin (1 μm) sections were collected on glass slides and stained with Toluidine Blue. In order to analyze PDL levels (M1 furcation area) of all the samples with TEM, we used Toluidine Blue staining to orient the images so that both the bucco-mesial (MB) and distal (DB) roots of the 1st molar were visible.
RNA isolation, reverse transcription and qRT-PCR
Total RNA was isolated from the PDL of 10-week-old Mkx+/+ and Mkx−/− mice using Isogen (Nippongene, Tokyo, Japan). Briefly, all the molars of upper and lower sides were pulled out and immediately immersed in ISOGEN. The molar samples from at least two mice were pooled and RNA was extracted. However, in 12-month-old mice (Fig. 1D), the maxillary region including three molars and surrounding alveolar bone were extracted as one lump and frozen with liquid nitrogen. Lumps were then ground with a mortar and RNA was extracted. Reverse transcription and qPCR were performed using ReverTra Ace (Toyobo, Osaka, Japan) and Thunderbird Master Mix (Toyobo), respectively. The expression of Gapdh was used as an internal control. Each sample was analyzed in triplicate, and the results were confirmed by at least three independent experiments. Primer sequences used for qPCR are listed in Table S1.
TRAP (tartrate-resistant acid phosphatase) staining
For TRAP staining, maxillary periodontal tissues of 12-month-old mice were fixed with 4% PFA/PBS at 4°C overnight and decalcified with 17.7% EDTA (Osteosoft, Merck, Tokyo, Japan) for 4 weeks. Then, paraffin sections were dehydrated, embedded and sectioned. Each section was stained using the TRAP Stain kit (Wako) according to the manufacturer's instructions.
Microarray analysis
RNA samples of 10-week- and 4-week-old mice PDL were prepared as described above. Microarray analysis was performed using Agilent Sure Print G3 Mouse Gene Expression arrays (8×60 K v2, Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer's instructions.
Cell culture
hPDL fibroblasts (Clonetics CC-7049 HPdLF, Lonza, Walkersville, MD, USA) were maintained at subconfluence using SCGM BulletKit (CC-3205, Lonza) at 37°C in a humidified 5% CO2 incubator.
The adenoviruses expressing either FLAG-Mkx or lacZ were generated with the Invitrogen ViraPower Adenoviral System (Thermo Fisher Scientific, Waltham, MA, USA) and purified using an Adeno-X Maxi Purification Kit (Clontech Laboratories, Mountain View, CA, USA). Titration of adenoviruses was determined using the Adeno-X Rapid Titer Kit (Clontech Laboratories). Seventy-two hours after infection, cells were collected and RNA was extracted.
To silence MKX expression, vectors for MKX siRNA (cat. no. SI04222064 Hs_MKX_3 FlexiTube siRNA, Qiagen, Hilden, Germany) or a negative control (cat. no. SI03650318, Qiagen) were used. The hPDL fibroblasts were transfected with Lipofectamine RNAiMax transfection reagent (Life Technologies, Thermo Fisher Scientific). At 48-72 h hours after transfection, RNA was extracted and purified.
qPCR was performed with specific primers for human genes as listed in Table S1.
Western blotting
Whole-cell lysates were prepared from FLAG-Mkx-expressing or control hPDL fibroblasts using RIPA buffer supplemented with 10 mM NaF, 1 mM Na3VO4, and protease inhibitor cocktail. The samples were subjected to western blot analysis with mouse anti-FLAG M2 antibody (F1804, Sigma-Aldrich, St Louis, MO, USA), mouse anti-β-actin antibody (AC-74, Sigma) for primary antibodies, and anti-mouse IgG-HRP for secondary antibody.
Statistical analysis
All data are expressed as the mean±s.e.m. The two-tailed independent Student's t-test was used to determine the level of significance. The probability level accepted for significance was P<0.05. Asterisks in figures indicate differences with statistical significance as follows: *P<0.05, **P<0.01 and ***P<0.001, throughout the manuscript.
Acknowledgements
We thank Prof Chisa Shukunami, Prof Yuji Hiraki, Dr Aki Takimoto and Dr Masayoshi Kawatsu for their technical advice in immunohistochemistry. We also thank Prof Sachiko Iseki, Dr Shoichi Suzuki, Dr Masafumi Inui, Dr Kenji Kobayashi, Dr Yuji Yamanishi and Dr Paveenarat Aukkarasongsup-Gondo for valuable discussions and Dr Tomoki Chiba for technical advice.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
N.K., M.S., T.S., K.M. and H.A. designed the study. N.K., T.S., S.I., K.K. and R.N. performed the experiments. N.K., Y.I., T.K., H.S. and K.M. analyzed data. N.K., T.S., M.S. and H.A. wrote the manuscript.
Funding
This research is (partially) supported by the Advanced Research and Development Programs for Medical Innovation from the Japan Agency for Medical Research and development (AMED), Japan Society for the Promotion of Science (JSPS) KAKENHI (26113008, 15H02560, 15K15540, 15K15544), and grants from the National Institutes of Health (AR050631, AR065379) and the Naito Foundation to H.A. Deposited in PMC for release after 12 months.
Data availability
Microarray data are deposited in NCBI Gene Expression Omnibus (GEO) with accession number GSE92331.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.135798.supplemental
References
- Ahuja T., Dhakray V., Mittal M., Khanna P., Yadav B. and Jain M. (2012). Role of collagen in the periodontal ligament - A Review. Internet J. Microbiol. 10, 1-7. [Google Scholar]
- Anderson D. M., Arredondo J., Hahn K., Valente G., Martin J. F., Wilson-Rawls J. and Rawls A. (2006). Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn. 235, 792-801. 10.1002/dvdy.20671 [DOI] [PubMed] [Google Scholar]
- Aubin J. E. and Bonnelye E. (2000). Osteoprotegerin and its ligand: a new paradigm for regulation of osteoclastogenesis and bone resorption. Medscape Women Health 11, 5 10.1007/s001980070028 [DOI] [PubMed] [Google Scholar]
- Aukkarasongsup P., Haruyama N., Matsumoto T., Shiga M. and Moriyama K. (2013). Periostin inhibits hypoxia-induced apoptosis in human periodontal ligament cells via TGF-beta signaling. Biochem. Biophys. Res. Commun. 441, 126-132. 10.1016/j.bbrc.2013.10.027 [DOI] [PubMed] [Google Scholar]
- Avery J. K. (2000). Essentials of Oral Histology and Embryology: A Clinical Approach, Japanese Edition. Japan: Nishimura Co., Ltd. [Google Scholar]
- Beertsen W., McCulloch C. A. G. and Sodek J. (1997). The periodontal ligament: a unique, multifunctional connective tissue. Periodontol. 2000 13, 20-40. 10.1111/j.1600-0757.1997.tb00094.x [DOI] [PubMed] [Google Scholar]
- Brent A. E., Schweitzer R. and Tabin C. J. (2003). A somitic compartment of tendon progenitors. Cell 113, 235-248. 10.1016/S0092-8674(03)00268-X [DOI] [PubMed] [Google Scholar]
- Chen F.-M. and Jin Y. (2010). Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng. B Rev. 16, 219-255. 10.1089/ten.teb.2009.0562 [DOI] [PubMed] [Google Scholar]
- Dangaria S. J., Ito Y., Luan X. and Diekwisch T. G. H. (2011). Successful periodontal ligament regeneration by periodontal progenitor preseeding on natural tooth root surfaces. Stem Cells Dev. 20, 1659-1668. 10.1089/scd.2010.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa D., Wada N., Maeda H., Yoshida S., Mitarai H., Tomokiyo A., Monnouchi S., Hamano S., Yuda A. and Akamine A. (2015). Wnt5a induces collagen production by human periodontal ligament cells through TGFbeta1-mediated upregulation of periostin expression. J. Cell Physiol. 230, 2647-2660. 10.1002/jcp.24950 [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Amizuka N., Takeshita S., Takamatsu H., Katsuura M., Ozawa H., Toyama Y., Bonewald L. F. and Kudo A. (1999). Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J. Bone Miner. Res. 14, 1239-1249. 10.1359/jbmr.1999.14.7.1239 [DOI] [PubMed] [Google Scholar]
- Ichinose S., Muneta T., Koga H., Segawa Y., Tagami M., Tsuji K. and Sekiya I. (2010). Morphological differences during in vitro chondrogenesis of bone marrow-, synovium-MSCs, and chondrocytes. Lab. Invest. 90, 210-221. 10.1038/labinvest.2009.125 [DOI] [PubMed] [Google Scholar]
- Ito Y., Toriuchi N., Yoshitaka T., Ueno-Kudoh H., Sato T., Yokoyama S., Nishida K., Akimoto T., Takahashi M., Miyaki S. et al. (2010). The Mohawk homeobox gene is a critical regulator of tendon differentiation. Proc. Natl. Acad. Sci. USA 107, 10538-10542. 10.1073/pnas.1000525107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruzynska-Frejtag A., Wang J., Maeda M., Rogers R., Krug E., Hoffman S., Markwald R. R. and Conway S. J. (2004). Periostin is expressed within the developing teeth at the sites of epithelial-mesenchymal interaction. Dev. Dyn. 229, 857-868. 10.1002/dvdy.10453 [DOI] [PubMed] [Google Scholar]
- Liu W., Watson S. S., Lan Y., Keene D. R., Ovitt C. E., Liu H., Schweitzer R. and Jiang R. (2010). The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol. Cell. Biol. 30, 4797-4807. 10.1128/MCB.00207-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhang C., Zhu S., Lu P., Zhu T., Gong X., Zhang Z., Hu J., Yin Z., Heng B. C. et al. (2015). Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFbeta signaling pathway. Stem Cells 33, 443-455. 10.1002/stem.1866 [DOI] [PubMed] [Google Scholar]
- Ma D., Zhang R., Sun Y., Rios H. F., Haruyama N., Han X., Kulkarni A. B., Qin C. and Feng J. Q. (2011). A novel role of periostin in postnatal tooth formation and mineralization. J. Biol. Chem. 286, 4302-4309. 10.1074/jbc.M110.140202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Wada N., Fujii S., Tomokiyo A. and Akamine A. (2011). Periodontal ligament stem cells. Stem Cells Clin. Res. 25, 619-637. 10.5772/23749 [DOI] [Google Scholar]
- Morse A. (1945). Formic acid-sodium citrate decalcification and butyl alcohol dehydration of teeth and bones for sectioning in paraffin. J. Dent. Res. 24, 143-153. 10.1177/00220345450240030501 [DOI] [Google Scholar]
- Nakahara H., Hasegawa A., Otabe K., Ayabe F., Matsukawa T., Onizuka N., Ito Y., Ozaki T., Lotz M. K. and Asahara H. (2013). Transcription factor Mohawk and the pathogenesis of human anterior cruciate ligament degradation. Arthritis Rheum. 65, 2081-2089. 10.1002/art.38020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamichi R., Ito Y., Inui M., Onizuka N., Kayama T., Kataoka K., Suzuki H., Mori M., Inagawa M., Ichinose S. et al. (2016). Mohawk promotes the maintenance and regeneration of the outer annulus fibrosus of intervertebral discs. Nat. Commun. 7, 12503 10.1038/ncomms12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizuka N., Ito Y., Inagawa M., Nakahara H., Takada S., Lotz M., Toyama Y. and Asahara H. (2014). The Mohawk homeobox transcription factor regulates the differentiation of tendons and volar plates. J. Orthop. Sci. 19, 172-180. 10.1007/s00776-013-0485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otabe K., Nakahara H., Hasegawa A., Matsukawa T., Ayabe F., Onizuka N., Inui M., Takada S., Ito Y., Sekiya I. et al. (2015). Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J. Orthop. Res. 33, 1-8. 10.1002/jor.22750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page R. C. and Schroeder H. E. (1976). Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab. Invest. 34, 235-249. [PubMed] [Google Scholar]
- Rios H., Koushik S. V., Wang H., Wang J., Zhou H.-M., Lindsley A., Rogers R., Chen Z., Maeda M., Kruzynska-Frejtag A. et al. (2005). periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol. Cell. Biol. 25, 11131-11144. 10.1128/MCB.25.24.11131-11144.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios H. F., Ma D., Xie Y., Giannobile W. V., Bonewald L. F., Conway S. J. and Feng J. Q. (2008). Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J. Periodontol. 79, 1480-1490. 10.1902/jop.2008.070624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo B.-M., Miura M., Gronthos S., Bartold P. M., Batouli S., Brahim J., Young M., Robey P. G., Wang C. Y. and Shi S. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364, 149-155. 10.1016/S0140-6736(04)16627-0 [DOI] [PubMed] [Google Scholar]
- Severson J. A., Moffett B. C., Kokich V. and Selipsky H. (1978). A histologic study of age changes in the adult human periodontal joint (ligament). J. Periodontol. 49, 189-200. 10.1902/jop.1978.49.4.189 [DOI] [PubMed] [Google Scholar]
- Sloan P. (1979). Collagen fibre architecture in the periodontal ligament. J. R. Soc. Med. 72, 188-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan P. C. D. (1995). Structural Organization of the Fibers of the Periodontal Ligament. London: Mosby-Wolfe. [Google Scholar]
- Suzuki H., Ito Y., Shinohara M., Yamashita S., Ichinose S., Kishida A., Oyaizu T., Kayama T., Nakamichi R., Koda N. et al. (2016). Gene targeting of the transcription factor Mohawk in rats causes heterotopic ossification of Achilles tendon via failed tenogenesis. Proc. Natl. Acad. Sci. USA 113, 7840-7845. 10.1073/pnas.1522054113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto A., Kawatsu M., Yoshimoto Y., Kawamoto T., Seiryu M., Takano-Yamamoto T., Hiraki Y. and Shukunami C. (2015). Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development 142, 787-796. 10.1242/dev.116228 [DOI] [PubMed] [Google Scholar]
- Uchibe K., Shimizu H., Yokoyama S., Kuboki T. and Asahara H. (2012). Identification of novel transcription-regulating genes expressed during murine molar development. Dev. Dyn. 241, 1217-1226. 10.1002/dvdy.23808 [DOI] [PubMed] [Google Scholar]
- Ueda-Maeda M. (2006). [The expression of transcription factor Osterix in human periodontal ligament cells]. Kokubyo Gakkai Zasshi 73, 62-69. 10.5357/koubyou.73.62 [DOI] [PubMed] [Google Scholar]
- Yokoi T., Saito M., Kiyono T., Iseki S., Kosaka K., Nishida E., Tsubakimoto T., Harada H., Eto K., Noguchi T. et al. (2007). Establishment of immortalized dental follicle cells for generating periodontal ligament in vivo. Cell Tissue Res. 327, 301-311. 10.1007/s00441-006-0257-6 [DOI] [PubMed] [Google Scholar]