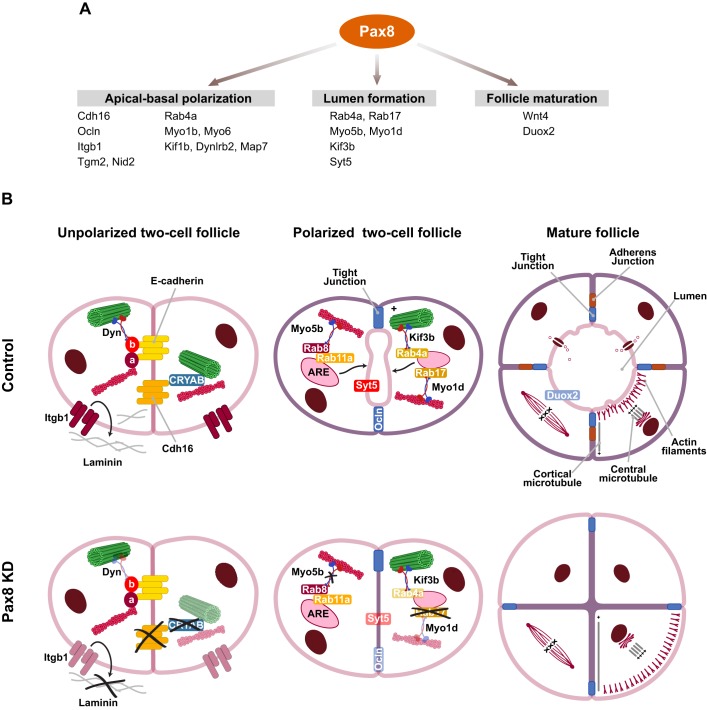
Fig. 6.
Pax8 is a regulator of 3D follicular polarization. (A) Summary diagram showing some of the Pax8 target genes that are enriched in 3D follicle-like structures and, here, classified according to their role in apical–basal thyroid cell polarization, lumen formation and follicle maturation. (B) Schematic model illustrating the Pax8-dependent follicle polarization and lumenogenesis process in vitro. A two-cell unpolarized aggregate presents loose cell–cell contacts rich in cadherins that bind to actin filaments through α-catenin and to microtubules through a β-catenin–dynein complex (‘a’ and ‘b’ in the image denote α- and β-catenin, respectively). Cadherin-16 (Cdh16) binds to actin filaments and to microtubules through CRYAB. β1-integrins (Itgb1) localized at the cell membrane transduce signals from the extracellular matrix (ECM). Vectorial transport of vesicles carrying apical membrane cargo mediated by Rabs and motor proteins followed by their exocytosis at the cell–cell contact site gives rise to the apical domain and allows initiation of lumen formation. In the absence of Pax8 [shPax8, Pax8 knockdown (KD)], cells lack Cdh16 at the nascent adherens junction that in turn leads to the downregulation of Itgb1 and laminins, impairing basement membrane assembly and its interaction with cells. Additionally, loss of CRYAB inhibits actin-filament and microtubule linking at the contact site to guide the transport of vesicles. The absence of Myo5b and Rab17, and downregulation of other Pax8 targets that mediate apical traffic and membrane polarization (Rab4a, Myo1d, Kif3b, Syt5, Ocln and Duox2), impedes apical domain definition between the two cells. Consequently, apical components are localized at the periphery of the follicle, and lumen formation is impaired.