Fig. 2.
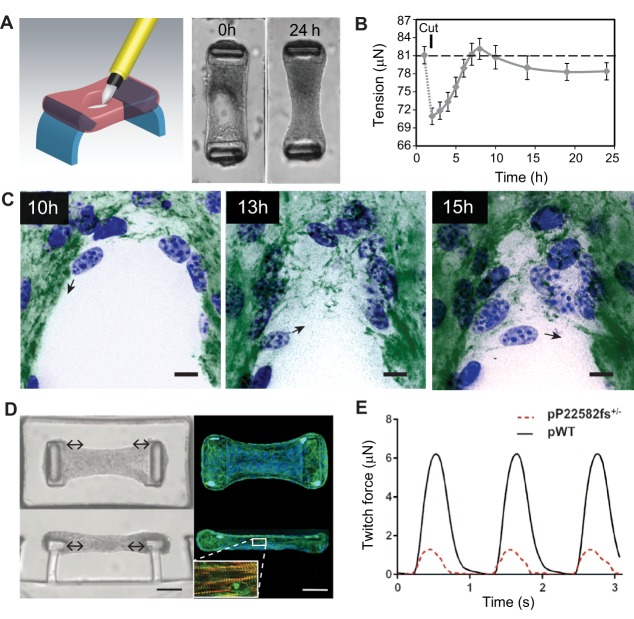
Application of constrained microtissues in wound healing and cardiac morphogenesis studies. Microtissues under tension exhibit entire tissue contractions and matrix remodeling during wound closure. (A) Using a microsurgical knife, full-thickness wounds are made in microtissues that are anchored to two flexible pillars (blue). Within 24 h, NIH/3T3 fibroblasts close the open gaps and restore tissue integrity. (B) Quantification of tissue contractility by measuring the deflection of the pillars shows that wound closure in microtissues is a staged process comprising relaxation after damage that is followed by tissue contraction and a steady-state sub-baseline tension stage. Error bars, s.e.m.; dashed line represents baseline tension before microtissue damage. (C) During the latter stage, fibroblasts (blue, nuclei) tow fibronectin (green) from the microtissue and assemble a provisional fibronectin template to bridge the gap, which serves as a substrate on which cells migrate further into the gap area (black arrows indicate migration direction of the cell). Scale bars: 25 µm. Panels A–C are adapted from Sakar et al., 2016 under CC BY 4.0 licence. In addition to wound healing, constrained microtissues have been employed to model cardiac function of human iPSC-differentiated cardiomyocytes. (D) Microscopy images [brightfield (left) and fluorescence (right; blue, nuclei; green, phalloidin)] of iPSC-cardiac microtissue (iPSC-CMT) suspended between two flexible pillars from top-down (upper) and side (lower) views. Upon maturation, iPSC-CMT forms aligned sarcomere structures (inset D; red, F-actin; green, α-actinin A) that beat synchronously and dynamically pull the caps of the pillars to the center of the tissue (double-headed arrows). Scale bars: 50 µm. (E) By measuring the deflection of the pillars, twitch force, a metric for cardiac function, can be assessed in iPSC-CMTs constituted from wild-type human iPSC-cardiomyocytes (pWT) or from individuals with mutations in titin, a structural sarcomere protein (pP22582fs+/−). Panels D and E are adapted from Hinson et al., 2015 with permission from AAAS.