Abstract
Rationale: In patients with chronic heart failure, daytime oscillatory breathing at rest is associated with a high risk of mortality. Experimental evidence, including exaggerated ventilatory responses to CO2 and prolonged circulation time, implicates the ventilatory control system and suggests feedback instability (loop gain > 1) is responsible. However, daytime oscillatory patterns often appear remarkably irregular versus classic instability (Cheyne-Stokes respiration), suggesting our mechanistic understanding is limited.
Objectives: We propose that daytime ventilatory oscillations generally result from a chemoreflex resonance, in which spontaneous biological variations in ventilatory drive repeatedly induce temporary and irregular ringing effects. Importantly, the ease with which spontaneous biological variations induce irregular oscillations (resonance “strength”) rises profoundly as loop gain rises toward 1. We tested this hypothesis through a comparison of mathematical predictions against actual measurements in patients with heart failure and healthy control subjects.
Methods: In 25 patients with chronic heart failure and 25 control subjects, we examined spontaneous oscillations in ventilation and separately quantified loop gain using dynamic inspired CO2 stimulation.
Measurements and Main Results: Resonance was detected in 24 of 25 patients with heart failure and 18 of 25 control subjects. With increased loop gain—consequent to increased chemosensitivity and delay—the strength of spontaneous oscillations increased precipitously as predicted (r = 0.88), yielding larger (r = 0.78) and more regular (interpeak interval SD, r = −0.68) oscillations (P < 0.001 for all, both groups combined).
Conclusions: Our study elucidates the mechanism underlying daytime ventilatory oscillations in heart failure and provides a means to measure and interpret these oscillations to reveal the underlying chemoreflex hypersensitivity and reduced stability that foretells mortality in this population.
Keywords: instability, loop gain, Cheyne-Stokes respiration, heart failure, chemosensitivity
At a Glance Commentary
Scientific Knowledge on the Subject
Oscillatory breathing during wakefulness predicts mortality in patients with heart failure, but the responsible mechanism is unclear. Associations with increased chemosensitivity and circulatory delay suggest instability of the chemoreflex feedback loop, but oscillatory patterns are often irregular, which illustrates that our knowledge is incomplete.
What This Study Adds to the Field
Our study provides the mechanism of daytime ventilatory oscillations in heart failure: ventilatory oscillations occur owing to a chemoreflex resonance or ringing effect, in which a reduced stability (increased loop gain)—due to increased chemosensitivity and delay—paradoxically enhances biological noise as it is propagated around the feedback loop, yielding stronger and more regular oscillations as stability is reduced. Our work may facilitate clinical measurement and interpretation of the oscillatory breathing that precedes sudden death in advanced heart failure.
The presence of daytime ventilatory oscillations is a powerful prognostic indicator of mortality in patients with chronic heart failure, independent of ejection fraction and peak oxygen consumption (1–6), but the underlying pathogenesis remains unclear. The feedback system controlling ventilation is strongly implicated based on evidence that patients with oscillatory ventilation exhibit hypersensitive ventilatory chemoreflexes and increased circulatory delays (5, 7, 8), and evidence that ventilatory oscillations are suppressed by interventions that improve stability (lowered loop gain), namely, reduced chemoreflex sensitivity, increased cardiac output, or clamped alveolar CO2 levels (5, 9–13). These findings have led to the prevailing view that feedback instability is responsible (7, 13–16), rather than a central pacemaker (17, 18). However, there is a broad spectrum of irregular oscillatory patterns observed in patients during wakefulness, many of which differ substantially from the remarkably consistent periodic cycles of apnea and the crescendo–decrescendo hyperpnea (Cheyne-Stokes respiration) that manifests during sleep and in computer models of feedback instability (16, 19). Thus, an alternative explanation for daytime ventilatory oscillations is needed.
According to prevailing theory, a hypersensitive and delayed ventilatory feedback system will yield ventilatory oscillations when the critical tipping point for instability is exceeded (loop gain > 1), but when the system is fundamentally stable, oscillations should be damped away (loop gain < 1; see Figures E1 and E2 in the online supplement) (7, 14, 16, 20). However, the instability theory has a critical weakness that precludes its general applicability: even stable feedback systems (loop gain <1) manifest a resonance or “ringing” effect in which random biological disturbances (e.g., intrinsic neural variability, sighs, and behavioral effects) repeatedly disturb the feedback loop, promoting temporary overshoot and undershoot oscillations with imprecise timing and amplitude (21–24). We propose that this concept underlays the pathogenesis of daytime ventilatory oscillations in patients with heart failure.
Here we assess whether ventilatory oscillations that occur during wakefulness are the consequence of a resonance in the chemoreflex feedback loop regulating ventilation. First, we describe and illustrate the concept of resonance as applicable to ventilatory oscillations. Subsequently, we assess daytime ventilatory oscillations in patients with heart failure and control subjects to test the hypothesis that the oscillatory behavior depends precisely on the stability (loop gain) of the ventilatory chemoreflex system (see the Theory subsection of the Methods section). Concordance with theory is taken to support chemoreflex resonance as the mechanism responsible. Preliminary data have been presented in abstract form (25).
Methods
Theoretical Basis of Resonance
The concepts of loop gain (i.e., stability) and resonance are well established, but the concept that loop gain precisely determines the strength of the resonance and the ensuing oscillatory nature of breathing under normal (stable) conditions has not been detailed previously (see the online supplement for details).
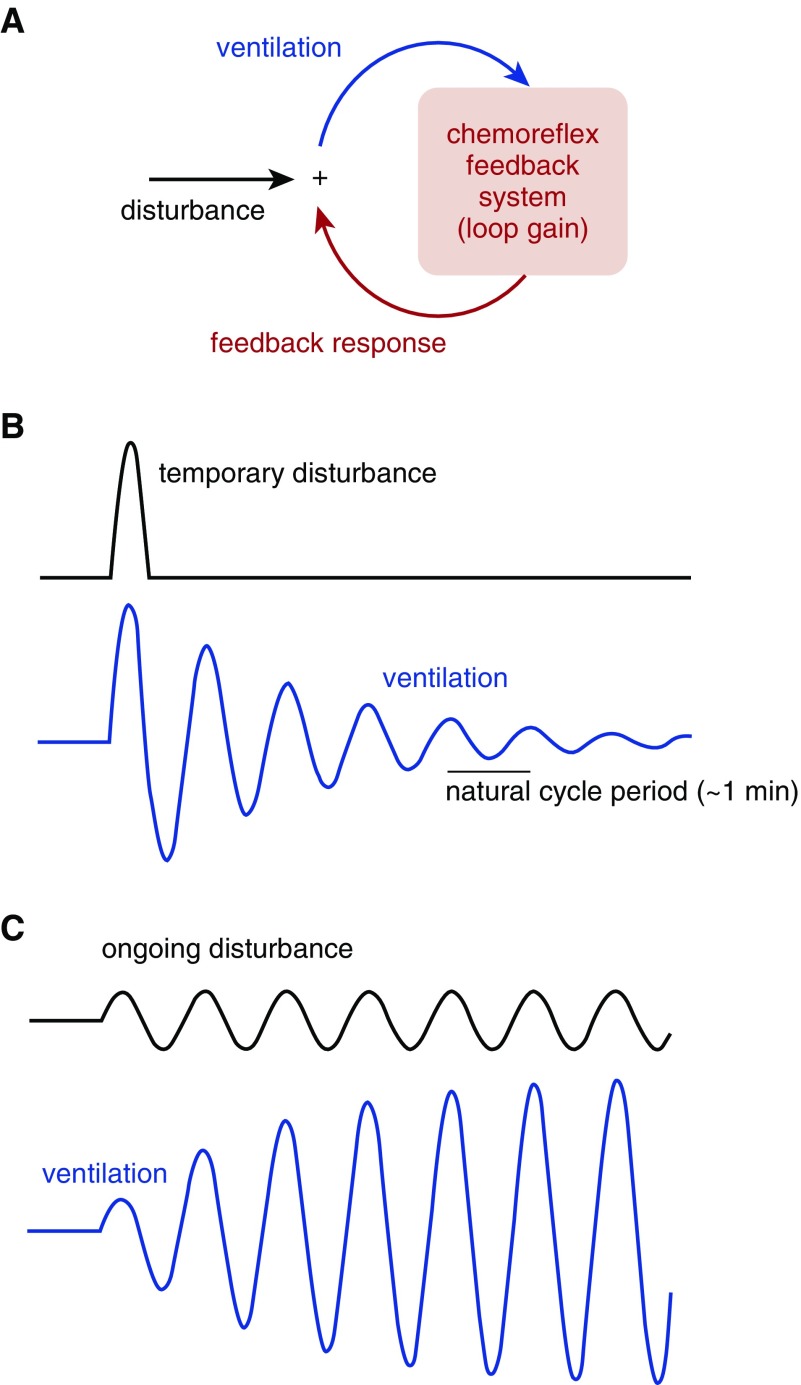
The stability of the chemoreflex feedback loop is determined by its loop gain, which is the ratio of the compensatory ventilatory feedback response that opposes a ventilatory disturbance (see conceptual model, Figure 1A). An isolated ventilatory disturbance provided to a stable system (loop gain = 0.8; Figure 1B) yields a oscillatory ringing effect at a particular frequency before gradually damping out. Yet, an ongoing disturbance at this frequency (akin to a child being pushed on a swing) produces ventilatory fluctuations that are considerably larger than the disturbance itself (Figure 1C). The ease by which ventilation fluctuates as a result of a disturbance (26–30) is determined by loop gain according to:
| (1) |
Figure 1.
Concept of chemoreflex resonance and the relationship with loop gain. (A) Feedback model for the chemoreflex regulation of ventilation. (B) In a stable system, a temporary disturbance that raises ventilation—thereby lowering alveolar carbon dioxide and later eliciting a reflex reduction in ventilatory drive—ultimately yields a resonance or ringing effect characterized by successive overshoot and/or undershoot fluctuations that damp out over time. Note that each feedback response (overshoot and/or undershoot) is approximately 0.8 times smaller than the previous deflection in ventilation (loop gain = 0.8). (C) In the same system, an ongoing disturbance is amplified to yield fivefold swings in ventilation, although feedback is stable (T = 5, see Equation 1).
where T defines the strength of the resonance and the strength of the ensuing oscillations. As loop gain rises toward 1 (i.e., the threshold for instability), feedback profoundly amplifies disturbances. For example, for a loop gain of 0.5, disturbances are doubled by the feedback system (T = 2); when loop gain is 0.8, disturbances are fivefold greater than they would be without feedback (T = 5; Figure 1C).
Simulated ventilatory oscillations
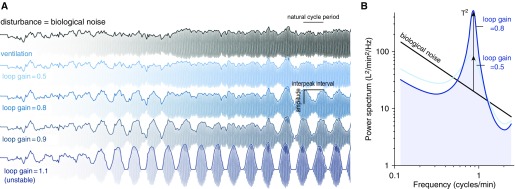
To illustrate the oscillatory characteristics that occur in the presence of spontaneous biological variations or “noise” (31), we examined a simple model system at various levels of loop gain (Figure 2). Note the distinct emergence of irregular oscillatory patterns (Figure 2A) that bear a remarkable resemblance to ventilatory patterns observed in patients with heart failure (13, 32, 33) and control subjects with experimentally raised loop gain (34) (see Results).
Figure 2.
Simulated chemoreflex oscillations. (A) A biological disturbance (top signal) is applied to ventilation for chemoreflex systems with increasing loop gain (reduced stability). Tidal breaths are drawn to faciliate comparison with ventilatory oscillations seen in patients with heart failure. (B) Spectral view of signals in A illustrates how biological noise is amplified by the system in a particular range of frequencies (near 1 cycle/min). In theory, the strength of the oscillation (T = amplitude/noise; vertical arrows) at the frequency of periodic breathing (“natural” cycle frequency) is determined by loop gain (Equation 1). Note also that slower disturbances are inhibited (reduced power at lower frequencies) as expected of homeostatic feedback (see the online supplement).
Importantly, we now recognize that as loop gain rises, a stronger resonance occurs that can be quantitatively identified as a stronger peak in the power spectrum of ventilation (Figure 2B), ultimately yielding larger and more regular oscillations.
Methodological Approach
Our primary objective was to test whether oscillatory strength, namely, amplitude relative to biological noise (i.e., T), is uniquely related to the loop gain of the ventilatory control system according to Equation 1. Loop gain was measured separately using dynamic inspired CO2 (see the following) during wakefulness. We also assessed whether larger amplitude, more regular oscillations are associated with a higher loop gain, and whether the spectral profile of oscillations matches that expected of a resonance.
Participants
Twenty-five patients with an established clinical diagnosis of chronic heart failure (any left ventricular ejection fraction) and 25 control subjects without heart failure were studied. Participants attended as part of larger ongoing prospective studies investigating the stabilizing mechanisms of acetazolamide and oxygen and the causes of sleep apnea (interventions were not given before and/or during this study). Inclusion required the absence of severe comorbidities, including lung, kidney, and liver diseases. Participants taking medications that affected respiratory control (including opioids, benzodiazepines, barbiturates, acetazolamide, theophylline, indomethacin, pseudoephedrine) were excluded. Participants provided written informed consent, and approval was granted by the Partners’ Institutional Review Board. Details are provided in the online supplement.
Procedure
Participants were examined by a physician before study procedures. Measurements were made in the morning (7 a.m.–12 p.m.) to minimize potential time-of-day effects. Participants were instrumented with a sealed nasal mask to facilitate measurement of ventilation (heated pneumotachograph and pressure transducer; Hans-Rudolph Model 3700, Kansas City, MO; Validyne Engineering Corp., Model MP45–14–871, Northridge, CA; ventilation = tidal volume × respiratory rate). Absence of mask leak was confirmed by forced expiration against a closed exhalation port. A thin catheter was placed through a port in the mask to measure intranasal CO2 tension (Pco2; Vacumetrics Inc., Model 17625, Ventura, CA) enabling assessment of inspired Pco2 and end-tidal Pco2 (a surrogate for alveolar and arterial Pco2). Electroencephalography (C3-A2, O2-A1) was performed to document wakefulness. Participants lay supine, and were instructed to relax, keep their eyes open and mouth closed (confirmed via visual assessment) and watched television as a distraction. Ventilation was recorded without interruption for 20 minutes to assess spontaneous ventilatory oscillations (see the following). Participants were subsequently connected to a non-rebreathing circuit for measurement of their chemoreflex stability (i.e., loop gain) using inspired CO2. For each procedure, a period of acclimation was provided to ensure ventilation and end-tidal Pco2 settled to an equilibrium before proceeding. Signals were sampled at 125 Hz (Power 1401 and Spike2, Cambridge Electronic Design Limited, Cambridge, UK); breath-by-breath respiratory signals were resampled at 4 Hz for further analyses.
Ventilatory Oscillations
To quantify the oscillatory nature of ventilation during spontaneous breathing, we performed spectral analysis and fit a physiological equation that describes the spectral profile of a resonance (Figure 2B; one-compartment delayed feedback stimulated by noise; see the online supplement). This analysis revealed a single parameter, T, which is a measure of the oscillatory strength (amplitude/background noise) that is theoretically related to loop gain (Equation 1). The peak-to-peak amplitude and irregularity (interpeak interval SD) of ventilatory oscillations were also quantified (see the online supplement).
Chemoreflex Stability
Loop gain was quantified with dynamic inspired CO2 stimulation using a modified method that used pulsatile CO2 stimuli. Seven percent–inspired CO2 was administered for 0.5 minutes, every 3 minutes, for a total of 30 minutes (10 pulses), which has the equivalent effect of stimulating ventilation at five frequencies simultaneously (0.33, 0.67, 1, 1.33, and 1.67 cycles/min). Chemosensitivity (Δventilation/Δalveolar Pco2), CO2 damping or plant gain (Δalveolar Pco2/Δventilation), and accompanying delays were calculated at each frequency to determine loop gain (chemosensivity × plant gain; see the online supplement).
Statistics
Linear regression assessed the relationship between the oscillatory strength (T, spectral analysis) and the underlying loop gain (CO2 stimulation). Oscillatory strength was first transformed (1 − 1/T, reflecting the estimated loop gain) before statistical analysis; transformed data became normally distributed, and correlations with putative physiological determinants became linear, as expected by theory. Fisher’s F tests compared the resonance model of the power spectrum versus the biological noise model without resonance within individuals; a significant improvement over noise confirmed the presence of a resonance (i.e., T significantly > 1). Student’s t tests compared variables between patients with heart failure and the control subjects; general linear models compared variables adjusted for age, sex, and body mass index (see the online supplement for matched comparisons). Determinants of loop gain, including chemoreflex sensitivity and delay, were quantified at a common frequency (1 cycle/min) for regression analyses; multiple regression results were summarized by presenting the improvement in the model r2 with the inclusion of each determinant in a sequential manner (forward stepwise). Unless specified otherwise, loop gain refers to the value at the natural frequency. Statistical significance was accepted at P < 0.05.
Results
Characteristics
Participant characteristics are detailed in Table 1. The patients with heart failure exhibited a range of severities of left ventricular ejection fraction (ejection fraction range: 15–67%; two individuals had preserved ejection fraction). All patients with heart failure were on optimal medical therapy per the attending cardiologist.
Table 1.
Patient Characteristics
| Characteristic | Heart Failure (n = 25) | Controls (n = 25) |
|---|---|---|
| Male:female, n | 23:2 | 15:10* |
| Age, yr | 61 ± 13 | 53 ± 13 |
| Body mass index, kg/m2 | 31 ± 7 | 32 ± 7 |
| Systolic dysfunction, yes:no, n | 23:2 | — |
| Left-ventricular ejection fraction, % | 38 ± 15 | 60 ± 3†‡ |
| New York Heart Association class, I:II:III, n | 3:13:8 | — |
| Medications, n (%) | ||
| β-Blockers | 24 (96) | 0 (0)* |
| Loop diuretics | 17 (68) | 0 (0)* |
| ACEi or AT2R blockers | 23 (92) | 2 (8)* |
| Spironolactone | 9 (36) | 0 (0)* |
| Digoxin | 6 (24) | 0 (0)* |
Definition of abbreviations: ACEi = angiotensin-converting enzyme inhibitor; AT2R = angiotensin type II receptor.
Values are mean ± SD unless otherwise indicated.
P < 0.05 (Fisher’s exact test).
Measured in a subset of 5 of 26 control subjects (and all patients with heart failure).
P < 0.001 patients with heart failure versus control subjects (Student’s t test).
Chemoreflex Stability
Assessment of chemoreflex feedback control of ventilation is detailed in Table 2. Patients with heart failure exhibited stable ventilatory control systems during wakefulness (loop gain range: 0.10–0.84) and exhibited a 71% higher loop gain than control subjects (P = 0.003, adjusted for age, sex, and body mass index).
Table 2.
Chemoreflex Stability
| Characteristic | Heart Failure (n = 25) | Controls (n = 25) |
|---|---|---|
| Summary | ||
| Loop gain | ||
| Mean ± SD | 0.43 ± 0.21 | 0.25 ± 0.09* |
| Range | 0.10–0.84 | 0.06–0.45 |
| Natural frequency, cycles/min | ||
| Mean ± SD | 1.33 ± 0.39 | 1.85 ± 0.51 |
| Range | 0.78–2.57 | 1.15–2.63 |
| Loop gain determinants† | ||
| Chemoreflex sensitivity, L/min/mm Hg‡ | 0.59 ± 0.24 | 0.48 ± 0.20§ |
| Plant gain, mm Hg/L · min‡ | 0.89 ± 0.21 | 0.99 ± 0.23 |
| Chemoreflex delay, s|| | 18.2 ± 4.6 | 13.8 ± 3.3¶ |
| Plant delay, s|| | 7.9 ± 1.4 | 8.2 ± 1.6 |
Values are mean ± SD unless otherwise indicated.
P < 0.001, patients with heart failure versus control subjects.
Values are reported for 1 cycle/min oscillations.
Chemoreflex sensitivity or controller gain describes the change in ventilation in response to a 1-mm Hg oscillation in alveolar Pco2. Plant gain describes the change in alveolar Pco2 caused by a 1 L/min oscillation in ventilation.
Nonsignificant trend (P = 0.08).
Chemoreflex delay describes the phase shift between alveolar Pco2 and ventilation (delay = phase lag/360° × 60) (7). This value reflects the lung-to-chemoreceptor circulation time plus additional time lags due to mixing of CO2 in the blood and tissues. Likewise, plant delay describes the phase shift between ventilation and alveolar Pco2 due to CO2 mixing in the lungs. Values are presented in units of time rather than phase to facilitate interpretation.
P < 0.01.
Ventilatory Oscillations
Example traces
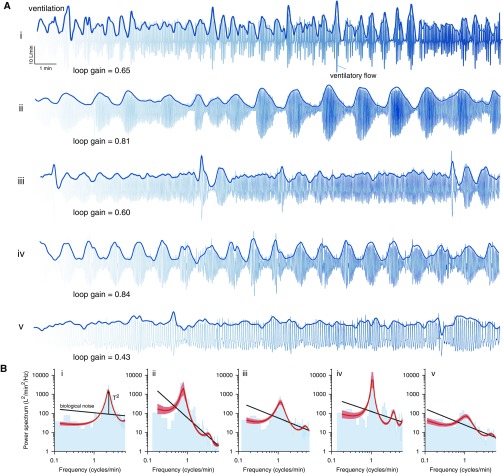
Ventilatory patterns during spontaneous breathing in five patients with heart failure are shown in Figure 3A. Note the profound, irregular oscillations bear a remarkable resemblance to the ventilatory oscillations emerging from feedback amplification of 1/f noise (Figure 3A vs. Figure 2A).
Figure 3.
Daytime ventilatory oscillations in patients with heart failure. (A) Ventilation data from five patients (i–v) are shown superimposed on ventilatory flow waveforms. (B) Corresponding power spectra are shown. Note the close fit of the resonance model (red lines, shading denotes SEM) to spectral data (blue bars). In theory, the strength of oscillations (amplitude/noise, T) is determined by the chemoreflex stability. Patients i and ii exhibited strong yet irregular overshoot–undershoot ventilatory oscillations. Patient iii exhibited modest oscillations after a transient disturbance (sigh breaths). Patient iv exhibited strong yet periodic oscillations consistent with instability (loop gain near 1). To the eye, patient v exhibited no overt oscillatory behavior in A, but spectral analysis reveals a weak oscillation (B). Amplitude in the scaling bar represents ventilation (tidal volume × respiratory rate).
Resonance model
The resonance model closely fit the measured spectral profile of ventilatory oscillations for each participant (see examples in Figure 3B and summary data in Table 3). The presence of a significant resonance was observed in 24 of 25 patients with heart failure and 18 of 25 control subjects (Fisher’s F test, which compared resonance to biological noise without feedback). Participants without a significant resonance (ventilatory variability resembled noise) tended to have a lower loop gain (see the online supplement).
Table 3.
Ventilatory Oscillations
| Characteristic | Heart Failure (n = 25) | Controls (n = 25) |
|---|---|---|
| Power spectral analysis of feedback amplification* | ||
| Oscillatory strength, T | ||
| Median (IQR) | 1.7 (1.2) | 1.4 (0.2)† |
| Range | 1.2–11.3 | 1.1–2.4 |
| Estimated loop gain, 1 − 1/T | 0.46 ± 0.19 | 0.29 ± 0.11† |
| Estimated natural frequency, cycles/min | 1.7 ± 0.5 | 2.5 ± 0.6† |
| Significant resonance detected‡, yes:no, n | 24:1 | 18:7§ |
| Time-domain analysis | ||
| Amplitude, % of mean | 47 (44) | 34 (23)‖ |
| Interpeak interval SD, % of mean | 26 ± 8 | 33 ± 6¶ |
Definition of abbreviation: IQR = interquartile range (75th percentile − 25th percentile).
Values are mean ± SD or median (IQR) unless otherwise indicated.
A resonance model was fit to the ventilation power spectrum to summarize the data. The general model is given by y = Sd(f)/|1 − LG(f)|2, where the noise component Sd(f) is assumed to conform to a power law [Sd(f) = βf−α, where α = exponent, β = offset, and f = frequency], and the chemoreflex influence is described by the simplest possible model [LG(f) = −ke−i2πfδ/(1 + i2πfτ), where k = gain,τ = time constant, and δ = delay] (41, 50).
P < 0.001.
Fisher’s F test compared the resonance model (feedback stimulated by biological noise) to noise (without feedback) for each individual.
P < 0.05, Fisher’s exact test.
‖P < 0.05.
P < 0.01.
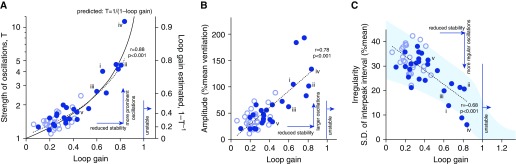
We observed a notable concordance between the oscillatory strength (T) seen using spectral analysis and the underlying loop gain taken from CO2 stimulation (Figure 4A), as expected from theory (Equation 1). That is, the underlying loop gain accurately explains the oscillatory nature of ventilation. Importantly, this association enabled loop gain to be estimated accurately from spontaneous oscillations (estimated loop gain = 1 − 1/T) (Figure 4A).
Figure 4.
Reduced chemoreflex stability explains ventilatory oscillations in patients with heart failure. With increasing loop gain, oscillations became (A) stronger relative to biological noise, (B) larger in amplitude, and (C) more regular. (A) The strength of oscillations (spectral height relative to background noise, T) closely matched that predicted from the loop gain of the chemoreflex system regulating ventilation (solid black line; Equation 1). Accordingly the estimated loop gain from the spectra closely matched the measured loop gain (error = 0.03 ± 0.09, mean ± SD). Shading in C denotes 95% prediction interval of simulated data. Solid circles denote patients with heart failure, and open circles denote control subjects. Patients i–v from Figure 3 are noted.
Consistent with prediction, increasing loop gain was associated with oscillations that were larger (Figure 4B) and had less irregular timing (smaller SD of interpeak interval; Figure 4C).
The period of spontaneous oscillations was also associated with the measured natural cycling period (1/[natural frequency] based on CO2 stimulation, r = 0.75; P < 0.001) consistent with feedback resonance.
Determinants of Reduced Stability and Oscillations
Linear regression models included the four loop gain determinants shown in Table 2.
Determinants of chemoreflex stability
Across all participants, increased loop gain was explained by an increase in chemoreflex sensitivity (univariate r2 = 0.42; P < 0.001), chemoreflex delay (univariate r2 = 0.14; multiple regression Δr2 = 0.24; P < 0.001), and plant gain (i.e., reduced lung volume; univariate r2 < 0.01; multiple regression Δr2 = 0.13; P < 0.001).
Determinants of ventilatory oscillations
A stronger resonance (T, spectral analysis) was associated with increased chemoreflex sensitivity (univariate r2 = 0.36; P < 0.001), plant gain (univariate r2 < 0.01; multiple regression Δr2 = 0.15; P < 0.001), and circulatory delay (univariate r2 = 0.07; multiple regression Δr2 = 0.14; P < 0.001). The presence and/or absence of heart failure explained a minor additional component (Δr2 = 0.03; P < 0.001), which suggested that factors related to heart failure beyond the determinants reported had a minor independent impact. Oscillatory amplitude and irregularity were also explained by chemoreflex sensitivity and delays (see the online supplement).
Discussion
Our study elucidates the mechanism underlying daytime ventilatory oscillations, a key predictor of mortality in patients with heart failure (1–6). We found that reduced stability (increased loop gain)—consequent to increased chemosensitivity, delay, and plant gain—yields stronger oscillations precisely as expected based on the theoretical concept of resonance (Equation 1). Specifically, the chemoreflex feedback system regulating ventilation paradoxically enhances biological noise near the frequency of periodic breathing to yield overshoot and undershoot ventilatory oscillations. These ventilatory oscillations in heart failure are typically irregular (Figure 3A) and conform to a model of feedback resonance in 96% of patients (Figure 3B). As loop gain rises toward 1, oscillations become larger and more regular (Figures 2 and 4), yielding prominent periodic breathing despite being classed as a stable system according to classic criteria (loop gain < 1). In contrast to current understanding, the more extreme conditions of feedback instability are therefore not necessary for ventilatory oscillations to occur in heart failure (7, 13–16). Overall, our data are remarkably consistent with chemoreflex resonance as the predominant mechanism responsible. Our work therefore provides the field with a validated framework for interpreting and quantifying the broad range of oscillatory ventilatory behaviors seen commonly in patients with heart failure.
Comparison with Available Evidence
By linking the clinical pattern of ventilatory oscillations to the function of the chemoreflex feedback system that regulates ventilation, we provide a unifying explanation for a host of previous empirical findings. Observational studies consistently demonstrate associations between daytime oscillatory breathing in heart failure and factors that promote a less stable feedback regulation of ventilation, namely, increased chemosensitivity and circulatory delay (7, 8, 12). Interventions that diminish feedback act to suppress oscillations, which are seen as a reduced variability and the disappearance of a peak in the power spectrum of ventilation (5, 9–11, 13). In healthy individuals and animals breathing spontaneously, experimental studies have demonstrated associations between ventilatory fluctuations and previous swings in ventilation and Pco2, which are dependent on intact chemosensitivity (22, 26, 35). Modeling studies have also suggested that a stronger chemoreflex response or higher loop gain yields quasi-oscillations in the presence of biological noise (24), although a quantitative relationship between oscillatory behavior and reduced stability had not been proposed or tested experimentally until now. Taken together with the present study, the available evidence now overwhelmingly implicates chemoreflex feedback regulation in the ventilatory oscillations observed.
Physiological Insights
Several key insights can be drawn from our work: Based on the concept of resonance, some degree of ventilatory oscillations must occur as a necessary side effect of homeostatic regulation. Specifically, a greater chemoreflex sensitivity will more completely suppress a long-term or steady-state disturbance to ventilation (e.g., a change in respiratory mechanics or metabolic rate), but will yield a greater amplification of biological noise at its characteristic frequency (see Figure 2B; also see the online supplement). The greater circulatory delay that occurs in heart failure will increase the amplification at the resonance, but it also moves the resonance to a lower frequency where biological noise is greater.
Oscillations result from chemoreflex feedback across a stability–instability continuum. Individuals with very low loop gain (e.g., 0 < loop gain ≤ 0.25) exhibit a pattern that resembles biological noise. Those with normal loop gain (0.25 < loop gain ≤ 0.5) exhibit weak and irregular oscillations. Patients with elevated loop gain (0.5 < loop gain ≤ 1) manifest stronger and more regular oscillations (Figure 3). Finally, consistent periodic breathing occurs in the most extreme cases when the threshold for instability is breached (loop gain > 1).
When the loop gain is below 1, the magnitude of biological noise plays a key role in the pathogenesis of oscillatory breathing. For example, in Figures 2 and 3, patients i and iii have quite similar loop gains, but patient i has twofold larger oscillations due to increased noise. Consequently, ventilatory fluctuations can be larger as a consequence of increased loop gain or increased noise. Thus, two distinct phenotypes of excessive ventilatory variability can be described: those driven largely by hypersensitive chemoreflex feedback (normal biological noise levels), and those with increased biological noise [i.e., ataxic opioid-induced ventilatory fluctuations (36) or ventilatory fluctuations in rapid-eye movement sleep (37)].
The concept of resonance has important implications for periodic breathing during sleep, known as central sleep apnea, which is also a strong prognostic marker of mortality in heart failure (1). Although sleep diminishes chemosensitivity per se, ventilatory oscillations become even more prominent (9). Key contributing factors include changes to state (sleep–wake transitions, arousals) and upper-airway patency (e.g., swings in dilator muscle tone) (38). Insofar as arousals and changes to upper-airway patency are tied to Pco2, such effects effectively raise loop gain by exacerbating changes in ventilation per change in Pco2. However, to the extent that arousals and upper airway effects are random, they provide an additional source of biological variability that will act to promote oscillatory breathing with maximum impact in those with elevated loop gain. Diminishing these disturbances with hypnotics and/or continuous positive airway pressure can improve central sleep apnea (39). Such disturbances may also explain residual events after loop gain is lowered to stable levels with intervention (40).
The concept also has implications for obstructive sleep apnea, a condition characterized by irregular ventilatory oscillations due to a combination of increased upper airway collapsibility and reduced ventilatory stability (41). Interestingly, reducing loop gain can improve obstructive sleep apnea severity even when the control system is strictly stable before intervention (41), which is potentially due to damping of chemoreflex resonance effects.
Clinical Implications
In patients with heart failure, increased chemosensitivity and consequent ventilatory oscillations are harbingers of the neurohumoral derangement that ultimately predisposes to mortality (42, 43). On this basis, a simple means to quantify reduced stability, as distinct from increased biological noise, may have clinical usefulness. Importantly, the present work enables a quantitative identification of the propensity to instability in individual patients from spontaneous breathing, without intervention. We and others have used spontaneous breathing to quantify stability (26, 41, 44, 45), but the use of a single variable to estimate stability without intervention has not been validated to date. Our approach may help (1) recognize the predisposition to Cheyne-Stokes respiration during wakefulness or sleep, (2) provide a means to titrate medications or screen those at high risk of sudden cardiac death, and (3) assess the impact of novel therapies designed to reduce chemosensitivity. Further investigation is warranted.
Limitations
Detailed mechanisms
Our study does not attempt to elucidate the specific chemoreceptors responsible for the ventilatory oscillations observed. Peripheral and central chemoreceptor systems may both contribute to the dynamic response measured with CO2 stimulation, although available evidence suggests an essential role for the carotid body chemoreceptors in the ventilatory oscillations and mortality in heart failure (46–49). Hypoxic chemosensitivity may also play a role (8), so including it in a measure of loop gain may further improve the associations observed. We also did not seek to elucidate the main source of ventilatory noise. Sources may be either extrinsic (e.g., behavioral inputs, neural variability external to chemoreflex feedback) or intrinsic (e.g., neural variability at the level of respiratory pattern generator or within chemoreceptor circuits in the medulla). The precise details of ventilatory disturbances were not under investigation; the essential point is that biological variability acts to disturb ventilation across a broad frequency range in all individuals.
End-tidal Pco2 as an estimate of alveolar and arterial Pco2
End-tidal Pco2 is used ubiquitously in ventilatory control studies of patients with and without heart failure to reflect breath-to-breath changes to alveolar and arterial Pco2. Particular care was taken to ensure a sufficient plateau, such that end-tidal Pco2 reflected alveolar levels (see the online supplement). Moreover, we excluded patients with lung disease; nonetheless, the difference between end-tidal and arterial Pco2 may be considerable in some patients with heart failure (e.g., via subclinical pulmonary congestion). We note, however, that a constant discrepancy between these two variables will have no impact on the values of loop gain measured because this calculation depends on relative Pco2 changes rather than the absolute value.
Nonlinearities
The resonance concept used here can be considered a linear simplification of more general nonlinear behavior. We note that spectral analysis of the oscillation traces revealed subtle higher harmonics at multiples of the natural frequency (i.e., not explained by the linear resonance model) in 3 of 25 patients with heart failure and 0 of 25 control subjects, which is consistent with the absence of nonlinear effects except in extreme cases (see patients ii and iv in Figure 3; note the smaller peaks not explained by the red model trace; see the online supplement).
Conclusions
Using a combination of mathematical modeling and direct measurement in patients with heart failure, our study demonstrates that daytime breathing oscillations in heart failure are readily explained by a potent resonance or ringing effect due to the chemoreflex feedback system regulating ventilation. Reduced stability—consequent to increased chemosensitivity and delay—leads to a greater amplification and propagation of biological noise around the feedback loop, yielding transient overshoot and undershoot oscillations that become profound as stability is reduced. We may now decipher oscillatory characteristics to more readily detect and interpret the otherwise covert increases in chemoreflex sensitivity that are known to occur with advanced heart failure and foretell mortality.
Acknowledgments
Acknowledgment
The authors are grateful for the technical assistance from Alison Foster, Lauren Hess, Pamela DeYoung, and Erik Smales; for the medical assessments performed by Drs. Robert Owens, David McSharry, and Jeremy Beitler; for the facilitation of patient recruitment from Drs. Michael Givertz, James Januzzi, Anju Nohria, William Dec, Garrick Stewart, Eldrin Lewis, Leonard Lilly, and Lynne Stevenson; and for discussions with Drs. Tilo Winkler and Morgan Mitchell.
Footnotes
Supported by the American Heart Association (11POST7360012 and 15SDG25890059), National Institutes of Health (NIH) (K24HL093218-01A1, 1R01HL090897-01A2, 5R01HL048531-16, R01HL102321, and P01HL095491), National Health and Medical Research Council of Australia (NHMRC) (1053201), the Menzies Foundation, and the American Thoracic Society Foundation. This work was also supported by Harvard Catalyst (National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH Award UL1TR001102). S.A.S. was also supported by grants from the NHMRC (1038402 and 1064163) and NIH (R01HL128658). B.A.E. was supported by an NHMRC fellowship (1035115). A.M. was principal investigator on NIH grants R01HL085188 and K24HL132105 and coinvestigator on R21HL121794, R01HL119201, and R01HL081823.
Author Contributions: Conception and design: S.A.S., Y.M., B.A.E., S.N., A.S.D., D.P.F., and A.M. Mathematical framework: S.A.S., S.N., and J.P.B. Model simulations: S.A.S. and Y.M. Modified approach to assess stability: S.A.S. Data collection and analysis: S.A.S., B.A.E., A.W., and A.M. Drafted the manuscript S.A.S. and J.P.B. All authors interpreted data, edited the manuscript for important intellectual content, and approved the final draft.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201604-0761OC on August 25, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lanfranchi PA, Braghiroli A, Bosimini E, Mazzuero G, Colombo R, Donner CF, Giannuzzi P. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99:1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 2.Corrà U, Pistono M, Mezzani A, Braghiroli A, Giordano A, Lanfranchi P, Bosimini E, Gnemmi M, Giannuzzi P. Sleep and exertional periodic breathing in chronic heart failure: prognostic importance and interdependence. Circulation. 2006;113:44–50. doi: 10.1161/CIRCULATIONAHA.105.543173. [DOI] [PubMed] [Google Scholar]
- 3.Guazzi M, Raimondo R, Vicenzi M, Arena R, Proserpio C, Sarzi Braga S, Pedretti R. Exercise oscillatory ventilation may predict sudden cardiac death in heart failure patients. J Am Coll Cardiol. 2007;50:299–308. doi: 10.1016/j.jacc.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 4.Arena R, Myers J, Abella J, Peberdy MA, Pinkstaff S, Bensimhon D, Chase P, Guazzi M. Prognostic value of timing and duration characteristics of exercise oscillatory ventilation in patients with heart failure. J Heart Lung Transplant. 2008;27:341–347. doi: 10.1016/j.healun.2007.11.574. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Anker SD, Chua TP, Francis D, Banasiak W, Poole-Wilson PA, Coats AJ, Piepoli M. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure: clinical implications and role of augmented peripheral chemosensitivity. Circulation. 1999;100:2418–2424. doi: 10.1161/01.cir.100.24.2418. [DOI] [PubMed] [Google Scholar]
- 6.Brack T, Thüer I, Clarenbach CF, Senn O, Noll G, Russi EW, Bloch KE. Daytime Cheyne-Stokes respiration in ambulatory patients with severe congestive heart failure is associated with increased mortality. Chest. 2007;132:1463–1471. doi: 10.1378/chest.07-0121. [DOI] [PubMed] [Google Scholar]
- 7.Francis DP, Willson K, Davies LC, Coats AJ, Piepoli M. Quantitative general theory for periodic breathing in chronic heart failure and its clinical implications. Circulation. 2000;102:2214–2221. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- 8.Giannoni A, Emdin M, Poletti R, Bramanti F, Prontera C, Piepoli M, Passino C. Clinical significance of chemosensitivity in chronic heart failure: influence on neurohormonal derangement, Cheyne-Stokes respiration and arrhythmias. Clin Sci (Lond) 2008;114:489–497. doi: 10.1042/CS20070292. [DOI] [PubMed] [Google Scholar]
- 9.Fontana M, Emdin M, Giannoni A, Iudice G, Baruah R, Passino C. Effect of acetazolamide on chemosensitivity, Cheyne-Stokes respiration, and response to effort in patients with heart failure. Am J Cardiol. 2011;107:1675–1680. doi: 10.1016/j.amjcard.2011.01.060. [DOI] [PubMed] [Google Scholar]
- 10.Murphy RM, Shah RV, Malhotra R, Pappagianopoulos PP, Hough SS, Systrom DM, Semigran MJ, Lewis GD. Exercise oscillatory ventilation in systolic heart failure: an indicator of impaired hemodynamic response to exercise. Circulation. 2011;124:1442–1451. doi: 10.1161/CIRCULATIONAHA.111.024141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannoni A, Baruah R, Willson K, Mebrate Y, Mayet J, Emdin M, Hughes AD, Manisty CH, Francis DP. Real-time dynamic carbon dioxide administration: a novel treatment strategy for stabilization of periodic breathing with potential application to central sleep apnea. J Am Coll Cardiol. 2010;56:1832–1837. doi: 10.1016/j.jacc.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 12.Mortara A, Sleight P, Pinna GD, Maestri R, Capomolla S, Febo O, La Rovere MT, Cobelli F. Association between hemodynamic impairment and Cheyne-Stokes respiration and periodic breathing in chronic stable congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;84:900–904. doi: 10.1016/s0002-9149(99)00462-2. [DOI] [PubMed] [Google Scholar]
- 13.Pinna GD, Maestri R, Mortara A, La Rovere MT, Fanfulla F, Sleight P. Periodic breathing in heart failure patients: testing the hypothesis of instability of the chemoreflex loop. J Appl Physiol (1985) 2000;89:2147–2157. doi: 10.1152/jappl.2000.89.6.2147. [DOI] [PubMed] [Google Scholar]
- 14.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53:644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 15.Cherniack NS, Longobardo GS. Cheyne-Stokes breathing. An instability in physiologic control. N Engl J Med. 1973;288:952–957. doi: 10.1056/NEJM197305032881810. [DOI] [PubMed] [Google Scholar]
- 16.Sands SA, Edwards BA, Kee K, Turton A, Skuza EM, Roebuck T, O’Driscoll DM, Hamilton GS, Naughton MT, Berger PJ. Loop gain as a means to predict a positive airway pressure suppression of Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 2011;184:1067–1075. doi: 10.1164/rccm.201103-0577OC. [DOI] [PubMed] [Google Scholar]
- 17.Franklin KA, Sandström E, Johansson G, Bâlfors EM. Hemodynamics, cerebral circulation, and oxygen saturation in Cheyne-Stokes respiration. J Appl Physiol (1985) 1997;83:1184–1191. doi: 10.1152/jappl.1997.83.4.1184. [DOI] [PubMed] [Google Scholar]
- 18.Bartsch S, Haouzi P. Periodic breathing with no heart beat. Chest. 2013;144:1378–1380. doi: 10.1378/chest.12-2950. [DOI] [PubMed] [Google Scholar]
- 19.Milhorn HT, Guyton AC. An analog computer analysis of Cheyne-Stokes breathing. J Appl Physiol. 1965;20:328–333. [Google Scholar]
- 20.Nyquist H. Regeneration theory. Bell Syst Tech J. 1932;11:126–147. [Google Scholar]
- 21.Khoo MCK. Complex dynamics in physiological control systems. In: Herrick RJ, editor. Physiological control systems analysis, simulation, and estimation. Hoboken, NJ: John Wiley & Sons, Inc.; 2000. pp. 271–308. [Google Scholar]
- 22.Van den Aardweg JG, Karemaker JM. Influence of chemoreflexes on respiratory variability in healthy subjects. Am J Respir Crit Care Med. 2002;165:1041–1047. doi: 10.1164/ajrccm.165.8.2104100. [DOI] [PubMed] [Google Scholar]
- 23.Modarreszadeh M, Bruce EN. Ventilatory variability induced by spontaneous variations of PaCO2 in humans. J Appl Physiol (1985) 1994;76:2765–2775. doi: 10.1152/jappl.1994.76.6.2765. [DOI] [PubMed] [Google Scholar]
- 24.Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 25.Sands SA, Nemati S, Mebrate Y, Edwards BA, Manisty CH, Turton A, Wellman A, Willson K, Francis DP, Malhotra A. Ventilatory oscillations in stable control systems as an interaction between external disturbances and feedback stability[abstract] Sleep. 2012;35:A48. [Google Scholar]
- 26.Nemati S, Edwards BA, Sands SA, Berger PJ, Wellman A, Verghese GC, Malhotra A, Butler JP. Model-based characterization of ventilatory stability using spontaneous breathing. J Appl Physiol (1985) 2011;111:55–67. doi: 10.1152/japplphysiol.01358.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammer PE, Saul JP. Resonance in a mathematical model of baroreflex control: arterial blood pressure waves accompanying postural stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1637–R1648. doi: 10.1152/ajpregu.00050.2004. [DOI] [PubMed] [Google Scholar]
- 28.Nisbet RM, Gurney WSC. A simple mechanism for population cycles. Nature. 1976;263:319–320. doi: 10.1038/263319a0. [DOI] [PubMed] [Google Scholar]
- 29.Ogata K. Frequency response analysis. In: Robbins T, editor. Modern control engineering. 3rd ed. Upper Saddle River, NJ: Prentice-Hall, Inc.; 1997. pp. 471–608. [Google Scholar]
- 30.Bode H. Network analysis and feedback filter design. New York: D. Van Nostrand Company; 1945. [Google Scholar]
- 31.Mutch WA, Harms S, Ruth Graham M, Kowalski SE, Girling LG, Lefevre GR. Biologically variable or naturally noisy mechanical ventilation recruits atelectatic lung. Am J Respir Crit Care Med. 2000;162:319–323. doi: 10.1164/ajrccm.162.1.9903120. [DOI] [PubMed] [Google Scholar]
- 32.Garde A, Sörnmo L, Jané R, Giraldo BF. Breathing pattern characterization in chronic heart failure patients using the respiratory flow signal. Ann Biomed Eng. 2010;38:3572–3580. doi: 10.1007/s10439-010-0109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortara A, Sleight P, Pinna GD, Maestri R, Prpa A, La Rovere MT, Cobelli F, Tavazzi L. Abnormal awake respiratory patterns are common in chronic heart failure and may prevent evaluation of autonomic tone by measures of heart rate variability. Circulation. 1997;96:246–252. doi: 10.1161/01.cir.96.1.246. [DOI] [PubMed] [Google Scholar]
- 34.Wellman A, Malhotra A, Fogel RB, Edwards JK, Schory K, White DP. Respiratory system loop gain in normal men and women measured with proportional-assist ventilation. J Appl Physiol (1985) 2003;94:205–212. doi: 10.1152/japplphysiol.00585.2002. [DOI] [PubMed] [Google Scholar]
- 35.Khatib MF, Oku Y, Bruce EN. Contribution of chemical feedback loops to breath-to-breath variability of tidal volume. Respir Physiol. 1991;83:115–127. doi: 10.1016/0034-5687(91)90097-3. [DOI] [PubMed] [Google Scholar]
- 36.Farney RJ, Walker JM, Cloward TV, Rhondeau S. Sleep-disordered breathing associated with long-term opioid therapy. Chest. 2003;123:632–639. doi: 10.1378/chest.123.2.632. [DOI] [PubMed] [Google Scholar]
- 37.Rostig S, Kantelhardt JW, Penzel T, Cassel W, Peter JH, Vogelmeier C, Becker HF, Jerrentrup A. Nonrandom variability of respiration during sleep in healthy humans. Sleep. 2005;28:411–417. doi: 10.1093/sleep/28.4.411. [DOI] [PubMed] [Google Scholar]
- 38.Pinna GD, Robbi E, Pizza F, Caporotondi A, La Rovere MT, Maestri R. Sleep-wake fluctuations and respiratory events during Cheyne-Stokes respiration in patients with heart failure. J Sleep Res. 2014;23:347–357. doi: 10.1111/jsr.12109. [DOI] [PubMed] [Google Scholar]
- 39.Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009;5:122–129. [PMC free article] [PubMed] [Google Scholar]
- 40.Sands SA, Edwards BA, Kee K, Stuart-Andrews CR, Skuza EM, Roebuck T, Turton A, Hamilton GS, Naughton MT, Berger PJ.Control theory prediction of resolved Cheyne-Stokes respiration in heart failure Eur Respir J 2016. pii: ERJ-00615-2016 [DOI] [PubMed] [Google Scholar]
- 41.Terrill PI, Edwards BA, Nemati S, Butler JP, Owens RL, Eckert DJ, White DP, Malhotra A, Wellman A, Sands SA. Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015;45:408–418. doi: 10.1183/09031936.00062914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannoni A, Emdin M, Bramanti F, Iudice G, Francis DP, Barsotti A, Piepoli M, Passino C. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol. 2009;53:1975–1980. doi: 10.1016/j.jacc.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Ponikowski P, Chua TP, Anker SD, Francis DP, Doehner W, Banasiak W, Poole-Wilson PA, Piepoli MF, Coats AJ. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–549. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 44.Asyali MH, Berry RB, Khoo MC. Assessment of closed-loop ventilatory stability in obstructive sleep apnea. IEEE Trans Biomed Eng. 2002;49:206–216. doi: 10.1109/10.983454. [DOI] [PubMed] [Google Scholar]
- 45.Fleming PJ, Goncalves AL, Levine MR, Woollard S. The development of stability of respiration in human infants: changes in ventilatory responses to spontaneous sighs. J Physiol. 1984;347:1–16. doi: 10.1113/jphysiol.1984.sp015049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khoo MC, Yang F, Shin JJ, Westbrook PR. Estimation of dynamic chemoresponsiveness in wakefulness and non-rapid-eye-movement sleep. J Appl Physiol (1985) 1995;78:1052–1064. doi: 10.1152/jappl.1995.78.3.1052. [DOI] [PubMed] [Google Scholar]
- 47.Del Rio R, Marcus NJ, Schultz HD. Carotid chemoreceptor ablation improves survival in heart failure: rescuing autonomic control of cardiorespiratory function. J Am Coll Cardiol. 2013;62:2422–2430. doi: 10.1016/j.jacc.2013.07.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niewiński P, Janczak D, Rucinski A, Jazwiec P, Sobotka PA, Engelman ZJ, Fudim M, Tubek S, Jankowska EA, Banasiak W, et al. Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol. 2013;168:2506–2509. doi: 10.1016/j.ijcard.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162:2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 50.Wellman A, Eckert DJ, Jordan AS, Edwards BA, Passaglia CL, Jackson AC, Gautam S, Owens RL, Malhotra A, White DP. A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985) 2011;110:1627–1637. doi: 10.1152/japplphysiol.00972.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]