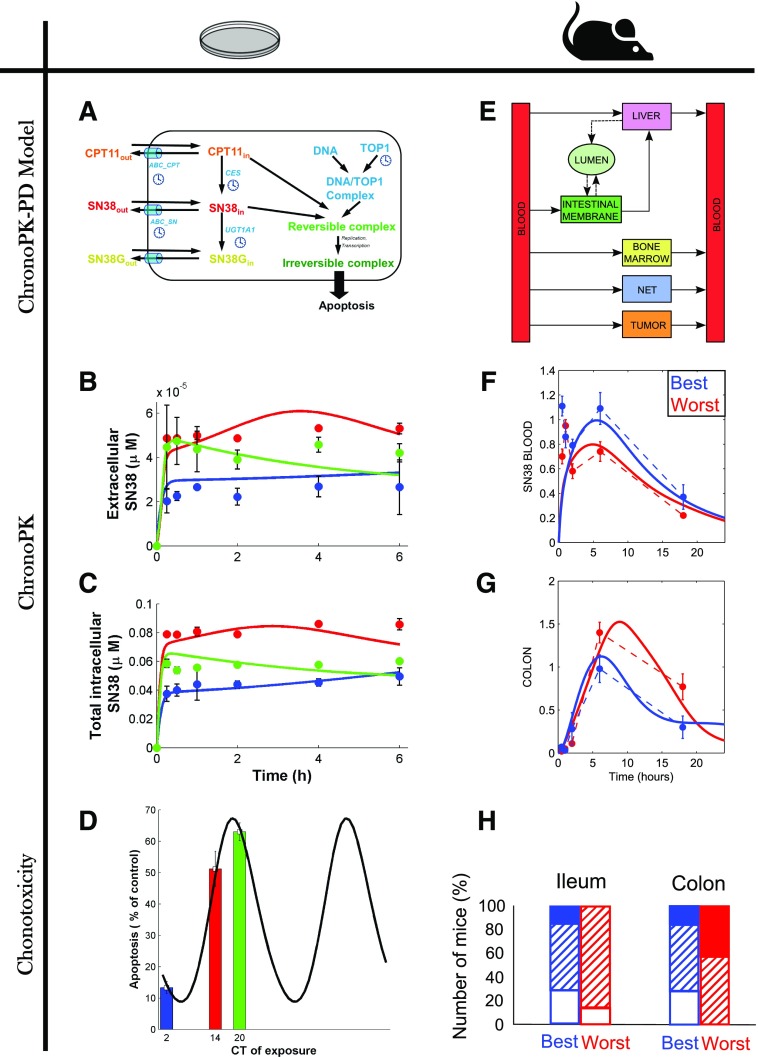
Fig. 7.
Multiscale systems chronopharmacology to personalize irinotecan chronotherapy. An in vitro study of irinotecan chronopharmacology led to the design of a cellular chronoPK-PD model (A) incorporating multitype experimental data, including the extra- and intracellular concentrations of active metabolite SN 38 and irinotecan-induced apoptosis after irinotecan exposure at three CTs (B–D) Dulong et al., 2015). This cellular investigation provided the basis for a mouse study and the development of a whole-body model of irinotecan chronoPK-PD explicitly incorporating the cellular model in relevant organs (E) (Ballesta et al., 2012). The model was first developed for B6D2F1 male mice in which several chronopharmacology datasets were available, including plasma and colon chronoPK profiles of SN38 after irinotecan at best and worst time of tolerability (F–G). The next step will consist in fitting intestinal chronotoxicity data available for the same mouse category (H) (Li et al., 2013). Dots or bars represent experimental results, and solid lines represent best-fit models.