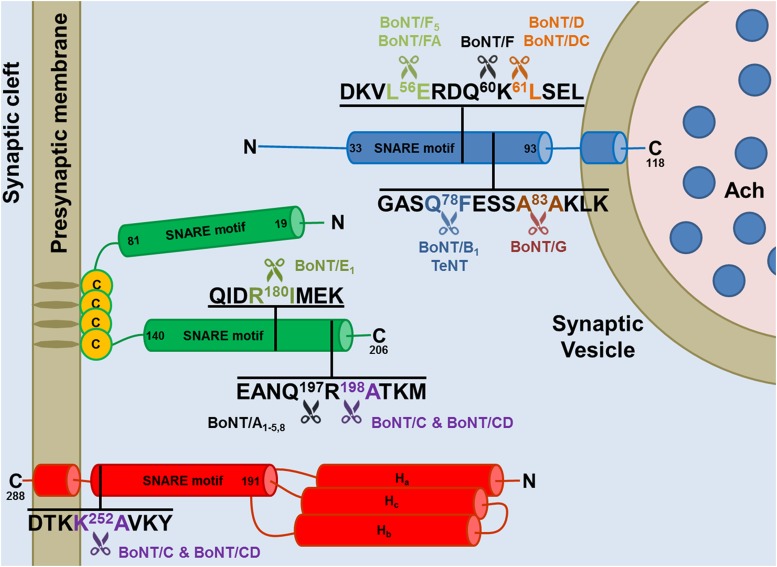
Fig. 3.
Cleavage sites of the neuronal SNARE proteins by the different BoNT types and subtypes. The BoNT proteolytic activity is highly specific and directed toward unique peptide bonds within the sequence of their respective SNARE protein targets. VAMP of the synaptic vesicle (in blue, isoform 1 is shown here) or SNAP-25 (in green) or syntaxin (in red, isoform 1B is shown here) mainly localized on the cytosolic side of the presynaptic membrane. Available evidence indicate that all the toxin subtypes and chimeric toxins cleave the same SNARE substrate, although different subtypes may cleave different peptide bonds. For example BoNT/F5 and BoNT/FA, a chimeric toxin derived from a genetic recombination between BoNT/F2, /F5, and A1 neurotoxin genes, cleave VAMP at a peptide bond different from the one cleaved by BoNT/F1. Notice that tetanus neurotoxin and botulinum B1 neurotoxin cleave the same target at the same site proving that the different symptoms of tetanus and botulism are not due to a different target molecule, but to different neuronal targets: the Renshaw cells of the spinal cord for tetanus neurotoxin and peripheral nerve terminals for BoNT/B1.