Over the past decade, the survival of patients with multiple myeloma (MM) has dramatically improved. This remarkable change is largely due to an increase in the anti-myeloma armamentarium, including next generation proteasome inhibitors (carfilzomib, ixazomib), next generation immunomodulatory drugs (IMiDs) (pomalidomide), and monoclonal antibodies (elotuzumab, daratumumab).1 However, the disease still remains incurable in the majority of cases and innovative strategies are, therefore, needed. BH3 mimetics represent a new class of drug that induce tumor cell death by targeting the anti-apoptotic proteins. Venetoclax, the first-in-class oral Bcl-2-specific BH3 mimetic, demonstrated impressive results in Bcl-2 dependent malignancies, such as chronic lymphocytic leukemia (CLL) and mantle cell lymphoma.2–4 We previously demonstrated that a subgroup of myeloma cells is Bcl-2 dependent, and therefore sensitive to venetoclax in vitro.5,6 Interestingly, sensitivity to venetoclax was found to be restricted to the myeloma cells harboring the translocation t(11;14).5 Here, we describe the case of a young patient with very advanced and refractory MM who achieved a deep and durable response after venetoclax therapy.
The patient is a 25-year old woman diagnosed with symptomatic IgG-λ MM in 2010 according to current International Myeloma Working Group (IMWG) criteria.7 At this time, she presented with symptomatic anemia. A bone marrow aspirate confirmed the presence of 27% plasma cells. The International Scoring System (ISS) score was intermediate (II). Fluorescence in situ hybridization (FISH) analysis revealed the presence of the translocation t(11;14) without other cytogenetic findings, such as t(4;14) or t(14;16), or 17p deletion. The therapies received by the patient are summarized in Figure 1. Disease response was assessed according to standard criteria.8 Front-line therapy consisted of 4 cycles of bortezomib-lenalidomide-dexamethasone (VRD) followed by high-dose melphalan and autologous stem cell transplantation (ASCT), and 2 consolidation cycles of VRD. After completion of therapy, the patient achieved a very good partial response (VGPR). She then received lenalidomide maintenance for one year from October 2011. The disease relapsed in January 2013 and the patient started lenalidomide-dexamethasone (Ld). The best response was only stable disease (SD) and the patient experienced a relapse in August 2015. At this time, FISH analysis revealed the presence of the 17p deletion in 76% of analyzed cells, in addition to the previously known translocation t(11;14). At that point, the triplet combination pomalidomide-bortezomib-dexamethasone (PVD) was started. After 2 cycles, the patient achieved a partial response (PR) but the disease progressed at the end of the fifth cycle. Next, she received daratumumab plus dexamethasone (IFM 2014-04 clinical trial), but the disease progressed at the end of the first cycle. We then decided to start carfilzomib in combination with bendamustine and dexamethasone. Again, the patient achieved a PR, but experienced early progression during cycle 3, with the development of a symptomatic right humeral lytic lesion.
Figure 1.
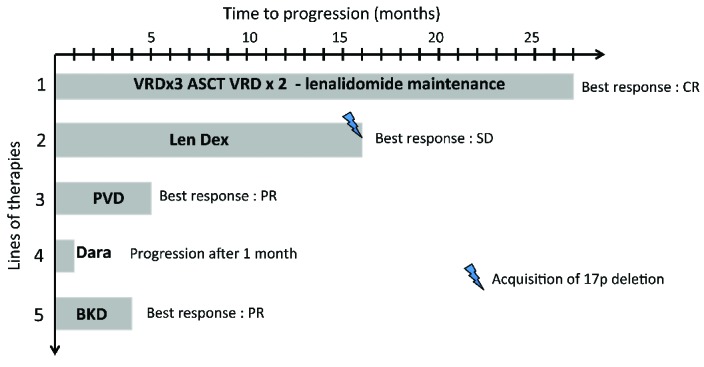
Prior lines of therapy before the initiation of venetoclax. VRD: bortezomib, lenalidomide, dexamethasone; ASCT: autologous stem cell transplantation; Len Dex: lenalidomide, dexamethasone; PVD: pomalidomide, bortezomib, dexamethasone; Dara: daratumumab; BKD: bendamustine, carfilzomib, dexamethasone; CR: complete response, PR: partial response; SD: stable disease.
In this situation of end-stage refractory MM, with the disease progressing on bortezomib, carfilzomib, lenalidomide, pomalidomide, bendamustine, dexamethasone and daratumumab, and harboring both t(11;14) and the 17p deletion, we approached Abbvie laboratories for a compassionate use of venetoclax. Both Abbvie and the French drug agency (Agence Nationale de Sécurité du Médicament, ANSM) gave their approval. In order to assess the plasma cell in vitro sensitivity, a new bone marrow sample was obtained after informed consent and plasma cells were cultured with increasing doses of venetoclax over 24 hours. Cell death was assessed using flow cytometry by measuring the loss of CD138 expression, as previously described.5 The plasma cells were found to be sensitive to venetoclax, with a lethal dose (LD)50 lower than 300 nM (Figure 2A). The patient started venetoclax (1200 mg/day) plus dexamethasone (40 mg/week) (Ven-Dex) in June 2016. At that time, she presented with anemia and symptomatic bone lesions. After one cycle of Ven-Dex, the M-spike decreased from 28 to 12 g/L (PR), bone pain disappeared and anemia was corrected without transfusion or use of any erythropoietin-stimulating agent. (Figure 2B) After 3 cycles, the patient achieved a VGPR. No drug-related adverse event has been reported so far. At the present time (December 2016), the patient is still receiving Ven-Dex and is still in VGPR (positive immunofixation, but M-spike not measurable). She presents no disease-related symptoms and describes her quality of life as “very good”.
Figure 2.
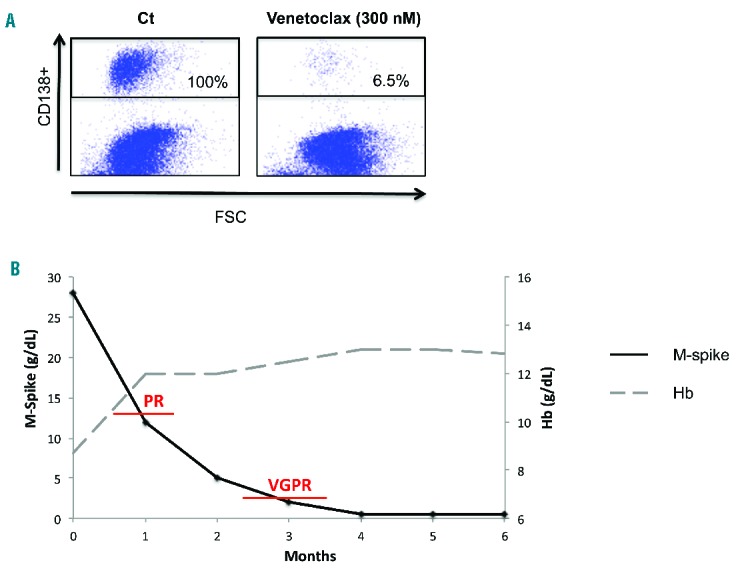
In vitro and clinical response to venetoclax. (A) Mononuclear cells were treated with 300nM of venetoclax for 24 hours. Cells were then stained with an anti-CD138-PE mAb. Plasma cell death was assessed by the loss of CD138 expression. Cell death percentage was calculated relative to control (ct) cells. FSC: forward scatter. (B) PR: partial response; VGPR: very good partial response.
The survival of patients with relapsed MM refractory to bortezomib and lenalidomide is very poor.9 In the relapsed setting, several novel agents, including pomalidomide, carfilzomib and daratumumab, have improved the prognosis of myeloma patients and have, therefore, been recently approved by the American and European drug agencies.10–12 Our patient reported here presented with end-stage myeloma refractory to bortezomib, lenalidomide, carfilzomib, pomalidomide and daratumumab. Moreover, she harbored a 17p deletion, a very high-risk cytogenetic feature. Despite these very poor characteristics, the patient achieved a deep (VGPR) and durable (at least 6 months) response after venetoclax therapy. For this patient, the duration of response to Ven-Dex was longer than that obtained with pomalidomide, carfilzomib and daratumumab. We previously demonstrated that in vitro sensitivity to venetoclax was restricted to MM cells harboring the translocation t(11;14).5 Here, we were able to test the in vitro sensitivity of the patient’s plasma cells to venetoclax. The LD50 was found to be in the nanomolar range, as previously observed for sensitive myeloma samples.5,6 Interestingly, this result confirms the fact that the alteration of the p53 pathway does not impact the sensitivity to venetoclax. Recently, a phase I dose-escalation trial evaluated the safety and efficacy of venetoclax as a single agent in relapsed myeloma patients.13 In the final 1200 mg/day cohort, the maximal tolerated dose was not reached, justifying the dose used for our patient reported here. In this trial, patients had very advanced disease with a median of 5 prior therapies. For patients with translocation t(11;14), the overall response rate (ORR) (at least PR) was 40%, including 6% CR and 20% VGPR. For patients without translocation t(11;14), almost no responses were observed (ORR: 6%). These results are very promising and demonstrate for the first time the clinical activity of a single agent in a specific cytogenetic subgroup of patients. Dexamethasone has been shown to promote Bcl-2 dependence in myeloma cells in vitro.14 Therefore, we chose to add dexamethasone to venetoclax, even if the patient was refractory to dexamethasone. In conclusion, the present case demonstrates the ability of venetoclax to induce deep and sustained responses in MM patients with translocation t(11;14), even in a setting of end-stage disease with high-risk 17p deletion.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge Abbvie for the supply of venetoclax.
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Moreau P, Touzeau C. multiple myeloma: from front-line to relapsed therapies. Am Soc Clin Oncol Educ Book. 2015:e504–511. [DOI] [PubMed] [Google Scholar]
- 2.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. [DOI] [PubMed] [Google Scholar]
- 3.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davids MS, Roberts AW, Anderson MA, et al. The BCL-2-Specific BH3-Mimetic ABT-199 (GDC-0199) Is Active and Well-Tolerated in Patients with Relapsed Non-Hodgkin Lymphoma: Interim Results of a Phase I Study. ASH Annu Meet Abstr. 2012;120(21):304. [Google Scholar]
- 5.Touzeau C, Dousset C, Le Gouill S, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014;28(1):210–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touzeau C, Ryan J, Guerriero J, et al. BH3 profiling identifies heterogeneous dependency on Bcl-2 family members in multiple myeloma and predicts sensitivity to BH3 mimetics. Leukemia. 2016;30(3):761–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–548. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17(8):e328–346. [DOI] [PubMed] [Google Scholar]
- 9.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. [DOI] [PubMed] [Google Scholar]
- 11.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016; 17(1):27–38. [DOI] [PubMed] [Google Scholar]
- 12.Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 2016;387(10027):1551–1560. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Vij R, Kaufman JL, et al. Phase I venetoclax monotherapy for relapsed/refractory multiple myeloma. J Clin Oncol. 2016;34(Suppl):abstract 8032. [Google Scholar]
- 14.Matulis SM, Gupta VA, Nooka AK, et al. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia. 2016;30(5):1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


