Figure 2.
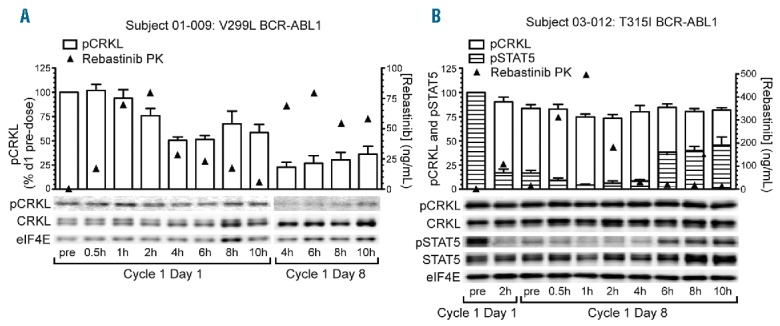
Pharmacodynamic analyses of pCRKL and pSTAT5 inhibition pre- and post-rebastinib dose. Serial peripheral blood samples were obtained from patients following the first dose of rebastinib (Cycle 1 Day 1) and on Day 8 of continuous twice daily dosing (Cycle 1 Day 8). Mononuclear cells were isolated, lysed, and the indicated proteins and phosphoproteins analyzed by immunoblotting as described in Methods. (A) Patient with relapsed chronic phase CML BCR-ABL1 V299L mutation who demonstrated >75% inhibition of pCRKL 4h following the Day 8 morning dose. (B) Patient with relapsed chronic phase CML and BCR-ABL1 T315I mutation who demonstrated >90% inhibition of pSTAT5 at 1h following the Day 8 morning dose. In this patient, the degree of inhibition of pCRKL was less pronounced (~25%). PK: pharmacokinetics; pCRKL: phosphorylated CT10 regulator of kinase-like; CRKL: CT10 regulator of kinase-like; eIF4E: eukaryotic translation initiation factor 4E; pSTAT5: phosphorylated signal transducer and activator of transcription 5; STAT5: signal transducer and activator of transcription 5.
