Abstract
Objective:
To examine the cross-sectional association between physical activity (PA) and hippocampal volume in middle-aged adults with childhood-onset type 1 diabetes (T1D), and whether hyperglycemia and insulin sensitivity contribute to this relationship.
Methods:
We analyzed neuroimaging and self-reported PA data from 79 adults with T1D from the Pittsburgh Epidemiology of Diabetes Complications Study (mean age 50 years, mean duration 41 years) and 122 similarly aged adults without T1D (mean age 48 years). Linear regression models, controlling for intracranial volume, sex, education, and age, tested associations between PA and gray matter volumes of hippocampi and total brain in the 2 groups. For the T1D group, models further controlled for hyperglycemia and glucose disposal rate, a measure of insulin sensitivity.
Results:
PA was significantly lower in the T1D than in the non-T1D group (median [interquartile range] 952 kcal [420–2,044] vs 1,614 kcal [588–3,091], respectively). Higher PA was significantly associated with larger hippocampi for T1D, but not for non-T1D (standardized β [p values] from regression models adjusted for intracranial volume, sex, age, and education: 0.270 [p < 0.001] and 0.098 [p = 0.12], respectively). Neither hyperglycemia nor glucose disposal rate substantially modified this association. Relationships between PA and total brain gray matter volume were similar.
Conclusions:
A cross-sectional association between higher PA and larger hippocampi is already detectable by middle age for these patients with T1D, and it appears robust to chronic hyperglycemia and insulin sensitivity. Proof-of-concept studies should investigate whether increasing PA preserves hippocampal volume and the mechanisms underlying the effects of PA on hippocampal volume.
Higher physical activity (PA) is increasingly recognized as a protective factor against gray matter atrophy, with strong effects in the hippocampus.1–3 Concerningly, only 35% of adults with type 1 diabetes (T1D) report being physically active,4,5 yet the relationship between PA and hippocampal volume has not been examined in this patient population. As people with T1D now live beyond middle age,6 it is critical to identify factors that could protect these patients from the combined effects of older age7 and hyperglycemia8 on hippocampal atrophy.
PA is associated with improved glycemic regulation in adults with and without diabetes, ages 40–60 years,9 possibly via improving insulin sensitivity.10 Poor insulin sensitivity has been shown to be related to hippocampal atrophy11 and dementia12 in people with and without diabetes. Therefore, lower PA could be related to smaller hippocampal volume in T1D, and hyperglycemia and insulin sensitivity could explain this association.
In this cross-sectional study, we examine the association between PA and hippocampal volume in middle-aged adults with and without childhood-onset T1D. We hypothesize that higher PA will be associated with larger hippocampi and that insulin sensitivity and hyperglycemia partially explain these relationships.
METHODS
Standard protocol approvals, registrations, and patient consents.
All study procedures received University of Pittsburgh's institutional review board approval and all participants provided informed consent prior to undergoing research procedures.
Participants.
Adults with T1D were recruited from the ongoing Pittsburgh Epidemiology of Diabetes Complications Study, an observational study of individuals diagnosed with childhood-onset T1D between 1950 and 1980, with the baseline assessment in 1986–1988 (n = 658, mean age 28 ± 8 years, mean T1D duration 19 ± 8 years). Participants completed biennial follow-up examinations and questionnaires through 1996–1998, and again in 2004–2006 (for details, see reference 13). Out of 263 locally dwelling (as of January 1, 2010) participants invited to this ancillary study, 26 never responded, 81 declined, and 2 were lost to follow-up. Of 154 interested, 37 were MRI-ineligible (e.g., metallic implants; for details, see reference 14), and 5 could not be scheduled. Of the 112 eligible and scheduled for neuroimaging, 3 did not show for their MRI and another 3 decided against the MRI at their scheduled examination. Including only those with data on physical activity (n = 93), insulin sensitivity (n = 96), depressive symptoms (n = 93), blood pressure (n = 102), and cholesterol (n = 104), concurrent with brain MRI, yielded an analytical sample of n = 79, mean age 50 ± 7 years, mean T1D duration 41 ± 6 years.
An observational study of associations between prehypertension and brain structure and function provided a comparison group of similarly aged adults without T1D. Parent study inclusion criteria were ages 35–60 years, local to Pittsburgh, and blood pressure <140/90 mm Hg without use of antihypertensive medication. Full exclusion criteria are provided elsewhere.14 Mailings/advertisements resulted in 414 people responding with interest; 110 were MRI-ineligible and 74 withdrew, leaving 230 enrolled. To mirror the racial distribution of the T1D cohort, only white participants with no missing data on physical activity (n = 130), depressive symptoms (n = 129), blood pressure (n = 130), and cholesterol (n = 125) were included, yielding an analytic sample of n = 122, mean age 49 ± 7 years.
MRI protocol.
In 2010–2013, all participants underwent brain MRI at the Pittsburgh Magnetic Resonance Research Center, using a Siemens (Munich, Germany) 12-channel head coil in a 3T Siemens Tim Trio scanner. Acquisition details are described in greater detail elsewhere.15,16 Magnetization-prepared rapid gradient echo T1-weighted images were acquired in the axial plane: repetition time 2,300 ms; echo time 3.43 ms; inversion time 900 ms; flip angle 90°; slice thickness 1 mm; field of view 256 × 224 mm; voxel size 1 × 1 mm; matrix size 256 × 240; number of slices 176.
Measures.
Gray matter volume.
FMRIB Software Library (FSL: fsl.fmrib.ox.ac.uk/fsl/fslwiki/) was used to segment gray matter, white matter, and CSF. The Montreal Neurologic Institute template was warped to the individual's MRI using a series of automated nonlinear registrations; this accounts for differences between the template and individual MRIs. After segmentation, all voxels classified as gray matter were summed to estimate total gray matter volume (mm3). Images were visually evaluated for inaccuracies or alignment problems. Our automated labeling technique, a method shown to be reliable and valid,17 was used to extract the gray matter volume of the hippocampus, as defined by the Montreal Neurological Institute anatomical brain template; this technique conducts atlas-based segmentation of MRI data by combining publicly available software (e.g., AFNI: afni.nimh.nih.gov/afni/) with customized programs. The hippocampus, including the dentate gyrus, the uncus, and the hippocampus proper, was demarcated in the sagittal view as the gray matter espousing the ventricular horns, and was limited caudally by the parahippocampal ramus of the collateral fissure.18 Volumes of left and right hippocampus were summed, providing hippocampi volume (mm3).
Total intracranial volume (mm3) was calculated as the sum of total brain volume and extraventricular CSF volume, after stripping the skull and meninges.
Physical activity.
All participants completed a modified, self-administered version of the Paffenbarger Physical Activity Questionnaire, concurrent with MRI. Weekly activity (kcal) was estimated using an algorithm based on stairs climbed, city blocks walked, and light, moderate, and vigorous activity.19
Demographic data (age, education, sex) were assessed concurrent with MRI.
Other risk factors related to hippocampal volume were assessed for all participants at time of MRI, using standardized methods: APOE4 allele20,21; depressive symptoms,20 per Beck Depressive Inventory score ≥1022 for participants with T1D and Center for Epidemiologic Studies–Depression scale score ≥1623 for participants without T1D; body mass index (BMI; kg/m2)20,21; blood pressure (mm Hg) and history of high blood pressure or using antihypertensive medications21; serum glucose (mg/dL)20; total cholesterol (mg/dL)21; and self-reported history of smoking 100 + cigarettes.20
T1D-specific factors.
The following were assessed as part of the T1D parent study at each physical examination, from baseline through 2010–2013, using standardized techniques (for details, see reference 13): T1D duration (years); estimated insulin sensitivity, as determined by a regression equation for estimated glucose disposal rate based on hyperinsulinemic-euglycemic clamps24; serum glucose (mg/dL); and long-term hyperglycemia per HbA1c months, an index of severity and duration of hyperglycemia.25 In addition, data on estimated glomerular filtration rate (mL·min−1·1.73 m−2) and the prevalence of proliferative retinopathy, distal symmetric polyneuropathy, and coronary artery disease were available from the parent study's 2004–2006 clinic examination, which occurred an average of 5 years prior to MRI (for details, see reference 13).
Statistical analyses.
Analysis of variance, Fisher exact, or Wilcoxon rank-sum score were used to compare participant characteristics by T1D status. Estimated PA (kcal/wk) was log10 transformed as it was highly skewed, and to improve the linearity assumption between PA and brain outcomes; all 0 values were changed to 0.05 prior to log-transformation.
Linear regression models examined the 2 cohorts combined, then each cohort separately. All models controlled for intracranial volume, to account for individual differences in head size, and models for the combined cohorts also controlled for T1D status. First, models tested whether PA (independent variable) was related to hippocampi (primary outcome) or total brain gray matter (secondary outcome) volumes, adjusting for age, education, and sex. Additional models further controlled for factors that differed by T1D status; these factors were selected after SIDAK correction for multiple comparisons, p < 0.004. For these models, the coefficient of PA predicting hippocampi or total brain gray matter volume was reported if statistically significant.
Linear regression models restricted to the T1D cohort tested the relationships between PA with hippocampi and with total brain gray matter volumes, controlling for intracranial volume, age, education, and sex. Models then tested whether adding HbA1c months or estimated glucose disposal rate one at a time, then combined, changed the coefficient of PA predicting hippocampi or total brain gray matter volume by >10%.26 Additional models further adjusted for factors that were significantly correlated to hippocampi per Spearman correlation, partialling out intracranial volume and age; these factors were selected after SIDAK correction for multiple comparisons, p < 0.003. Finally, all regression models restricted to the T1D cohort were repeated, controlling for T1D duration in place of age at MRI. To ease interpretation of the association between PA and hippocampi, we also repeated the models using raw PA (i.e., not log-transformed) to predict hippocampi.
Sensitivity analyses were performed using a subgroup of participants with no history of high blood pressure or stroke. In addition, to examine whether the association between PA and hippocampi was explained by total brain volume, we also adjusted for total brain volume; to address multicollinearity between intracranial volume and total brain gray matter volume, hippocampal volume normalized by intracranial volume was the outcome in these models.
Analyses were performed using SPSS (version 22.0; IBM Corp., Chicago, IL) and SAS 9.3 (SAS Institute, Cary, NC). Standardized β coefficients are reported for all models.
RESULTS
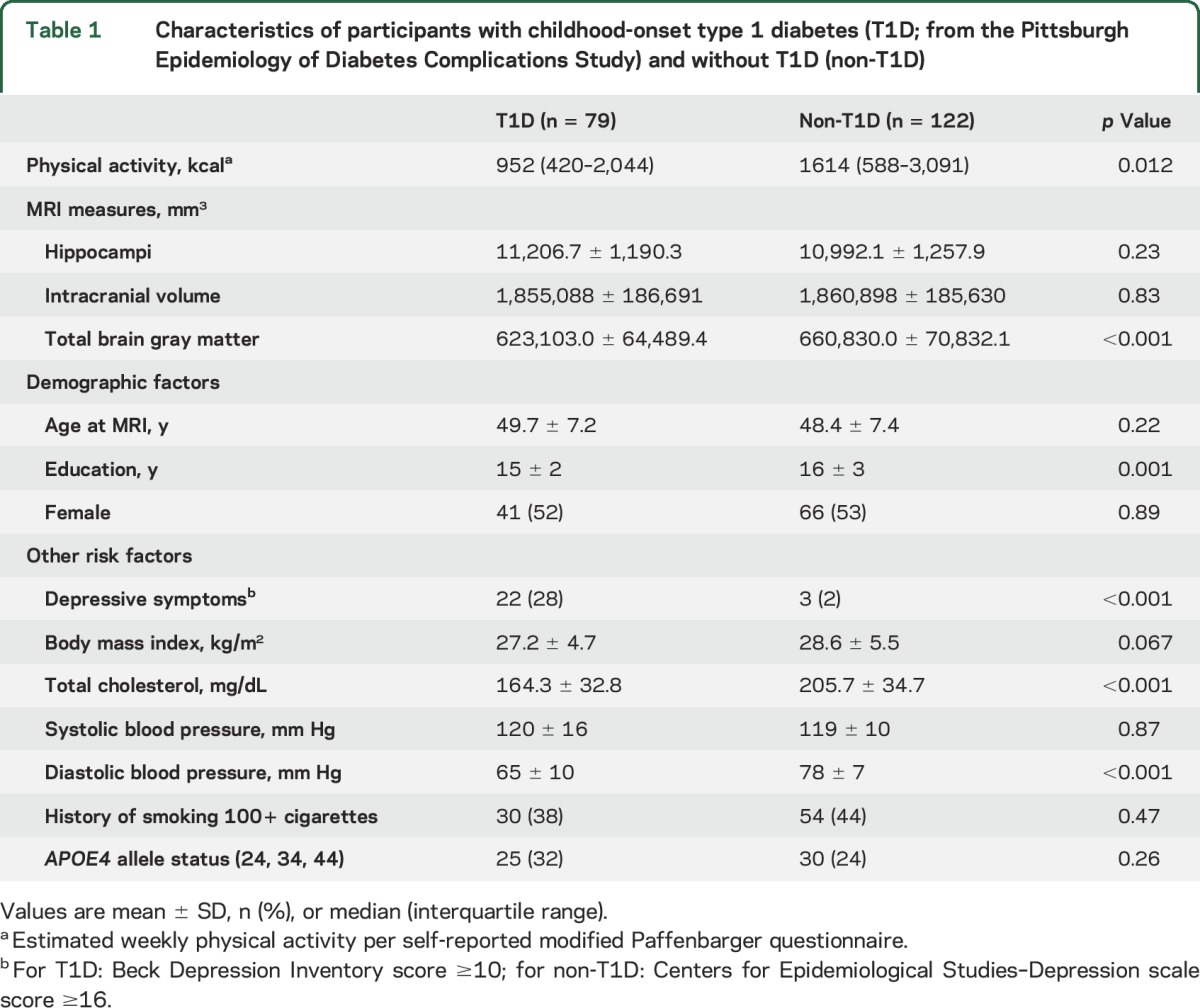
Characteristics of these middle-aged adults with and without T1D have been described in detail.14,27,28 PA was significantly lower among participants with T1D than among participants without T1D (table 1). The between-group difference in total brain gray matter volume was statistically significant, but differences in hippocampi or intracranial volume were not (table 1). Participants with T1D had statistically significantly fewer years of education, lower cholesterol, lower diastolic blood pressure, and higher prevalence of depressive symptoms than did participants without T1D (table 1, all p < 0.001). The cohorts did not differ in age, female:male distribution, BMI, systolic blood pressure, history of ever smoking, or APOE4 allele status (table 1; all p > 0.10).
Table 1.
Characteristics of participants with childhood-onset type 1 diabetes (T1D; from the Pittsburgh Epidemiology of Diabetes Complications Study) and without T1D (non-T1D)
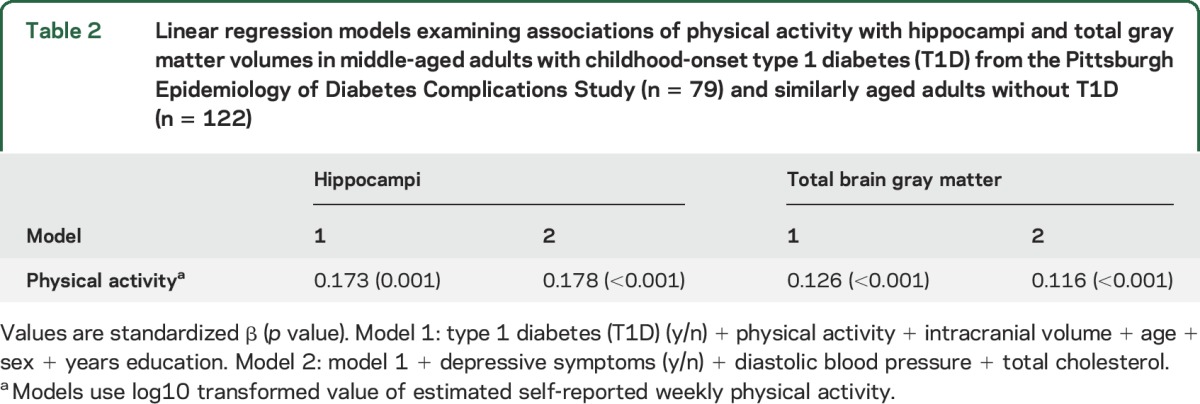
In linear regression models examining the 2 cohorts combined, higher PA was related to larger hippocampi and total brain gray matter volumes, controlling for T1D status, intracranial volume, sex, education, and age (table 2, model 1). Further adjustment for depressive symptoms, total cholesterol, and diastolic blood pressure only minimally modified the association between PA and hippocampi (table 2, model 2); these covariates were selected because they differed between groups per SIDAK correction (p < 0.004) (table 1).
Table 2.
Linear regression models examining associations of physical activity with hippocampi and total gray matter volumes in middle-aged adults with childhood-onset type 1 diabetes (T1D) from the Pittsburgh Epidemiology of Diabetes Complications Study (n = 79) and similarly aged adults without T1D (n = 122)
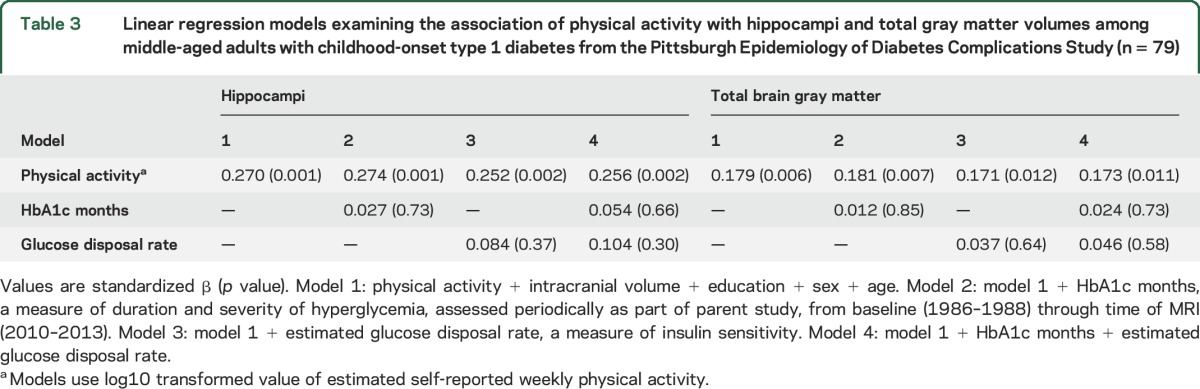
The association between PA and hippocampi was statistically significant for the T1D cohort (table 3, model 1). This association remained significant, and the coefficient of PA changed by <10%, when adjusting for chronic hyperglycemia and estimated glucose disposal rate (table 3, models 2–4), thus did not warrant formal mediation/moderation analyses.
Table 3.
Linear regression models examining the association of physical activity with hippocampi and total gray matter volumes among middle-aged adults with childhood-onset type 1 diabetes from the Pittsburgh Epidemiology of Diabetes Complications Study (n = 79)
In the model using raw PA (i.e., not log-transformed) to predict hippocampi, the unstandardized regression coefficient of each kcal/wk of PA was β = 0.126; thus, for each 1,000 kcal/wk (corresponding to about 30 minutes per day of brisk walking, 6 days a week), there would be a difference of 126 mm3 in hippocampi volume, which is approximately 1% of the mean hippocampal volume in this T1D cohort.
Associations between PA and total brain gray matter volume were similar to what was observed for hippocampi (table 3).
Associations between hippocampi and other T1D-specific factors were not significant above SIDAK correction for multiple comparisons; hence none was selected for further testing in the regression models (table e-1 at Neurology.org).
Results were overall similar when controlling for duration of T1D in place of age at MRI, or when controlling for total brain volume (not shown).
In models restricted to the non-T1D cohort, controlling for age, sex, education, and intracranial volume, PA was significantly associated with total gray matter volume, but not with hippocampi (standardized β [p values] = 0.100 [0.009]; 0.105 [0.096], respectively).
Results of these analyses were overall unchanged when we excluded participants with a history of high blood pressure (31 T1D, 10 non-T1D) or stroke (5 T1D) (data not shown).
DISCUSSION
In this T1D cohort, there was a cross-sectional association between lower PA and smaller hippocampi; this association was robust to adjustment for chronic hyperglycemia and a marker of insulin sensitivity, and also to several other well-known contributors of hippocampal atrophy, such as older age, depression,20 and APOE4 allele.20,21
While we cannot draw conclusions about causality due to study design, and despite advances in diabetes management and treatment since these individuals were diagnosed with T1D, our results hold potential relevance for this patient population. Specifically, for these adults with childhood-onset T1D, every 1,000 kcal expended per week corresponded to 126 mm3 larger hippocampi. In practical terms, these results could be appreciated as taking a brisk, 30-minute walk, 6 days per week, being related to 1.3% larger hippocampi. This difference is not negligible and could, in fact, potentially counteract the 1%/y decline in hippocampal volume estimated to occur among individuals age 55 years and older.7,29 As adults with T1D enter their 5th and 6th decades of life, preserving the hippocampus may be critical in efforts to reduce future risk of cognitive decline. Long-term exposure to T1D-related processes in adults ages 50 years and older is likely to negatively affect hippocampal volume, over and above effects of advancing age. If so, then middle age may be a most critical time for PA intervention to increase hippocampal volume in adults with T1D, before they progress into an even older age group, with a steeper rate of hippocampal atrophy.7
We also found that higher PA was related to larger total brain gray matter volume. Since T1D appears to negatively affect brain development early in the course of the disease, with effects evident on MRI shortly after T1D diagnosis,30 our finding suggests that higher PA may somewhat compensate for the sustained insults of childhood-onset T1D on total brain gray matter volume.
The associations between PA and hippocampal volume were independent of our measures of chronic hyperglycemia and insulin sensitivity. Animal models show that hyperglycemia may reduce hippocampal synaptic plasticity, neurogenesis, and differentiation.31,32 Higher glucose levels may also reduce hippocampal volume indirectly, via increasing levels of advanced glycation end products and reactive oxidative species.29,33 However, we did not find significant associations between measures of chronic hyperglycemia with hippocampal volume, similar to 3 prior studies examining children and young adults.34–36 In contrast, others report associations between hyperglycemia and smaller hippocampi, both cross-sectionally and longitudinally, in children and young adults.37,38
Even so, hyperglycemia and insulin sensitivity could still be in the pathway linking PA and hippocampal volume in T1D. It could be that increasing, or simply maintaining, PA over time, not PA measured at one time point, is associated with improved glycemic control, potentially via improved insulin sensitivity, and these factors may affect hippocampal volume over time.
Which other mechanisms could link higher PA to larger hippocampi in people with T1D? PA may act on molecules involved in angiogenesis and neurogenesis.1 For example, PA may stimulate neurogenesis by increasing levels of brain-derived neurotrophic factor. In addition, PA regulates insulin-like growth factor 1 and vascular endothelial growth factor (VEGF) production, molecules important for angiogenesis.1 Animal models show that PA induces capillary development in the hippocampi of mice and that blocking VEGF influx into the brain eliminates exercise-induced neurogenesis.1 This neurotrophic pathway could be of great importance for people with childhood-onset T1D, because T1D appears to reduce hippocampal neurogenesis and neuronal differentiation in animal models of T1D.31,32 Other possible pathways deserving investigation include PA potentially reducing the accumulation of advanced glycation end products and reactive oxidative species; stabilizing blood pressure; stabilizing the hypothalamus-pituitary-adrenal axis function; enhancing mood and the dopaminergic system; and reducing concentrations of inflammatory cytokines.33 It cannot be excluded that the associations observed would indicate a reverse relationship between hippocampus and PA; that is, lower hippocampal volume, via reduced cognition, would be related to reduced abilities to move around and be physically active.
The study's negative results also deserve mention. While PA was significantly related to larger hippocampi in our T1D cohort, the relationship was not significant for the cohort without T1D. It could be that the effects of PA on hippocampal volume are more noticeable among individuals with very low levels of PA, such as we observed in this T1D cohort, than among individuals with average PA levels for adults of this age group, such as we observed in our non-T1D cohort. Another possibility is that we only see an association between PA and hippocampus under conditions in which the brain is relatively impaired, such as in a disease like T1D. Second, T1D status was not significantly associated with hippocampal volume. Our null results are similar to prior case-control studies in children,34,36 young adults,38,39 and this middle-aged T1D cohort.27 In contrast, smaller hippocampal volumes were found cross-sectionally in a pediatric T1D population as compared with their nondiabetic peers.37 Likewise, several well-known contributors to hippocampal atrophy were not associated with hippocampi in this cohort. Most of our knowledge regarding the effects of T1D on hippocampal volume is derived from animal models of T1D31,32 and more work is needed to clarify these associations in humans with childhood-onset T1D.
Results of this study should be interpreted in the context of its limitations. PA was self-reported and this method, unlike objective accelerometry data, cannot fully capture physical activity. Even so, both cohorts utilized the same questionnaire and differential reporting by T1D status is unlikely. Some variables (e.g., proliferative retinopathy) were measured 5 years prior to MRI, possibly underestimating their true association with PA. Longitudinal studies with repeated neuroimaging would be especially valuable to examine the influence of PA changes on future changes in glycemic control, insulin sensitivity, and hippocampal volume. We previously reported that participants from the T1D parent study who underwent brain imaging are healthier than those who did not undergo brain imaging.28 While the parent studies of these 2 cohorts assessed depressive symptoms using different instruments, the cutpoints used are validated and have clinical relevance. Finally, the results of this study may not be generalizable to all people with T1D, especially not those diagnosed in adulthood, and a survival bias may exist for this T1D cohort. It cannot be excluded that changes over time in management of diabetes and cardiovascular complications may affect the relationship between PA and hippocampus. Future studies of patients with T1D with childhood onset, who will be reaching middle age in the next few decades, will be critical to assess to what extent the associations observed will differ. Although our findings need to be replicated, the cross-sectional association between PA and hippocampal volume in this T1D population underscores the importance of measuring the effect of increasing PA to preserve hippocampal and total brain gray matter volumes over time. Our findings lay the foundation for future intervention trials in T1D to examine whether increasing PA reduces change over time in hippocampal and total brain gray matter volumes, as well as the role of improved glycemic control over time on brain outcomes.
Supplementary Material
ACKNOWLEDGMENT
The author thanks the study participants.
GLOSSARY
- BMI
body mass index
- PA
physical activity
- T1D
type 1 diabetes
- VEGF
vascular endothelial growth factor
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
K.A.N. analyzed data, interpreted results, and drafted the manuscript. R.L.L. helped develop, review, and edit the manuscript. T.J.O. provided guidance of analyses development and helped revise the manuscript. T.C. provided guidance of analyses development and helped revise the manuscript. H.J.A. provided oversight and quality control of neuroimaging, data processing, and interpretation of findings, and helped revise the manuscript. J.R.J. oversaw collection and management of risk factor and neuroimaging data from the cohort without type 1 diabetes and helped revise the manuscript. K.I.E helped develop, review, and edit the manuscript. C.R. contributed to data analyses and helped with interpreting results and in writing the manuscript.
STUDY FUNDING
C.R. is the principal investigator of the neuroimaging study for the type 1 diabetes cohort (NIH grant: R01 DK089028). C.R. is the guarantor of the study and takes complete responsibility for the integrity of the data and accuracy of the data analysis. T.J.O. is the principal investigator of the EDC Study (NIH grant R37 DK034818-25), which provided risk factor and other data for the type 1 diabetes cohort. J.R.J. is the principal investigator of MR Hyper Study (NIH grant RO1 HL101959-05), which provided neurocognitive and other data on the cohort without type 1 diabetes.
DISCLOSURE
K. Nunley, R. Leckie, T. Orchard, and T. Costacou report no disclosures relevant to the manuscript. H. Aizenstein's research is funded in part by NIH grants MH076079, AG025516, AG037451, and AG044474. J. Jennings is funded by NIH grants: NHLBI 1RO1, NIMH 5RO1 MH084938, NIMH MH085722, NHLBI 5R01 HL105647, NHLBI 5R01 HL111802, NIMH 1R01 MH101088, NIA R01 AG014116. K. Erickson and C. Rosano report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci 2007;11:342–348. [DOI] [PubMed] [Google Scholar]
- 2.Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci 2006;61:1166–1170. [DOI] [PubMed] [Google Scholar]
- 3.Erickson KI, Raji C, Lopez O, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology 2010;75:1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas N, Alder E, Leese G. Barriers to physical activity in patients with diabetes. Postgrad Med J 2004;80:287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plotnikoff R, Taylor L, Wilson P, et al. Factors associated with physical activity in Canadian adults with diabetes. Med Sci Sports Exerc 2006;38:1526–1534. [DOI] [PubMed] [Google Scholar]
- 6.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of diabetes complications study cohort. Diabetes 2012;61:2987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser MA, Shaw ME, Cherbuin N. A systematic review and meta-analysis of longitudinal hippocampal atrophy in healthy human ageing. NeuroImage 2015;112:364–374. [DOI] [PubMed] [Google Scholar]
- 8.Weinstein G, Maillard P, Himali JJ, et al. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology 2015;84:2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dijk JW, Eijsvogels TM, Nyakayiru J, et al. Glycemic control during consecutive days with prolonged walking exercise in individuals with type 1 diabetes mellitus. Diabetes Res Clin Pract 2016;117:74–81. [DOI] [PubMed] [Google Scholar]
- 10.Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med 2000;21:1–12. [DOI] [PubMed] [Google Scholar]
- 11.Willette AA, Xu G, Johnson SC, et al. Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care 2013;36:443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hildreth KL, Van Pelt RE, Schwartz RS. Obesity, insulin resistance, and Alzheimer's disease. Obesity 2012;20:1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh epidemiology of diabetes complications study experience. Diabetes 2006;55:1463–1469. [DOI] [PubMed] [Google Scholar]
- 14.Nunley KA, Rosano C, Ryan CM, et al. Clinically relevant cognitive impairment in middle-aged adults with childhood-onset type 1 diabetes. Diabetes Care 2015;38:1768–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatraman VK, Aizenstein HJ, Newman AB, et al. Lower digit symbol substitution score in the oldest old is related to magnetization transfer and diffusion tensor imaging of the white matter. Front Aging Neurosci 2011;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006;148:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosano C, Becker J, Lopez O, et al. Morphometric analysis of gray matter volume in demented older adults: exploratory analysis of the cardiovascular health study brain MRI database. Neuroepidemiology 2005;24:221–229. [DOI] [PubMed] [Google Scholar]
- 18.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 19.Winters-Hart C, Brach J, Storti K, Trauth J, Kriska A. Validity of a questionnaire to assess historical physical activity in older women. Med Sci Sports Exerc 2004;36:2082–2087. [DOI] [PubMed] [Google Scholar]
- 20.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 2012;8:189–202. [DOI] [PubMed] [Google Scholar]
- 21.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol 2006;5:735–741. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988;8:77–100. [Google Scholar]
- 23.Lewinshon PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiological Studies–Depression scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 1997;12:277–287. [DOI] [PubMed] [Google Scholar]
- 24.Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes 2000;49:626–632. [DOI] [PubMed] [Google Scholar]
- 25.Orchard TJ, Forrest KZ, Ellis D, Becker DJ. Cumulative glycemic exposure and microvascular complications in insulin-dependent diabetes mellitus: the glycemic threshold revisited. Arch Intern Med 1997;157:1851–1856. [PubMed] [Google Scholar]
- 26.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 27.Hughes TM, Ryan CM, Aizenstein HJ, et al. Frontal gray matter atrophy in middle aged adults with type 1 diabetes is independent of cardiovascular risk factors and diabetes complications. J Diabetes Compl 2013;27:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunley KA, Ryan CM, Orchard TJ, et al. White matter hyperintensities in middle-aged adults with childhood onset type 1 diabetes. Neurology 2015;84:2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab 2016;36:172–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes 2014;63:2244–2252. [DOI] [PubMed] [Google Scholar]
- 31.Beauquis J, Saravia F, Coulaud J, et al. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol 2008;210:359–367. [DOI] [PubMed] [Google Scholar]
- 32.Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev 2013;37:1346–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrighten SA, Piroli GG, Grillo CA, Reagan LP. A look inside the diabetic brain: contributors to diabetes-induced brain aging. Biochim Biophys Acta 2009;1792:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aye T, Reiss AL, Kesler S, et al. The feasibility of detecting neuropsychologic and neuroanatomic effects of type 1 diabetes in young children. Diabetes Care 2011;34:1458–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes 2003;52:149–156. [DOI] [PubMed] [Google Scholar]
- 36.Hershey T, Perantie DC, Wu J, Weaver PM, Black KJ, White NH. Hippocampal volumes in youth with type 1 diabetes. Diabetes 2010;59:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marzelli MJ, Mazaika PK, Barnea-Goraly N, et al. Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes. Diabetes 2014;63:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Musen G, Lyoo IK, Sparks CR, et al. Effects of type 1 diabetes on gray matter density as measured by voxel-based morphometry. Diabetes 2006;55:326–333. [DOI] [PubMed] [Google Scholar]
- 39.Lobnig BM, Krömeke O, Optenhostert-Porst C, Wolf OT. Hippocampal volume and cognitive performance in long-standing type 1 diabetic patients without macrovascular complications. Diabet Med 2006;23:32–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


