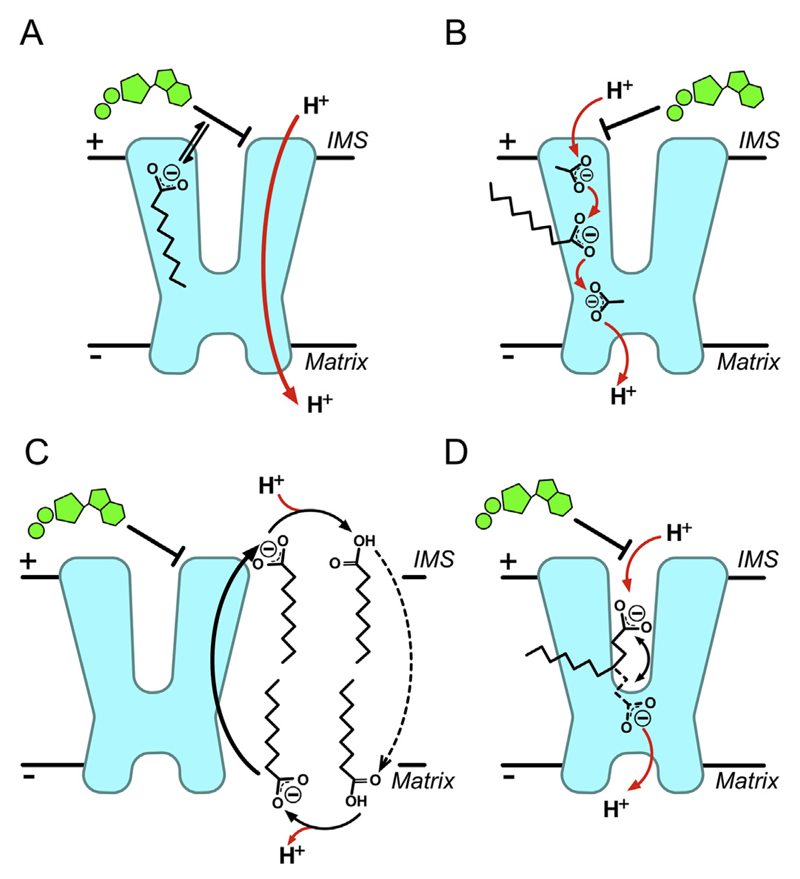
Fig. 2. Proposed mechanisms of proton transport by UCP1.
A) The functional competition model: fatty acids act simply to remove nucleotide inhibition of UCP1, either by direct competition or allosterically [45]. B) The cofactor model: fatty acids associate with UCP1 to provide carboxylate(s) as protonatable groups, which complete a proton transfer pathway within the protein [47]. C) The cycling model: fatty acid anions act as actual transport substrate of UCP1 and are exported, flipping back across the membrane in a protonated state independently of UCP1, to give net proton transfer [49,50]. D) The shuttling model: both protonated or deprotonated fatty acid species can be transported/exchanged by UCP1, however, a restricted ability to release hydrophobic long chain fatty acids from the protein results in net proton transfer only [57]. See main text for details.