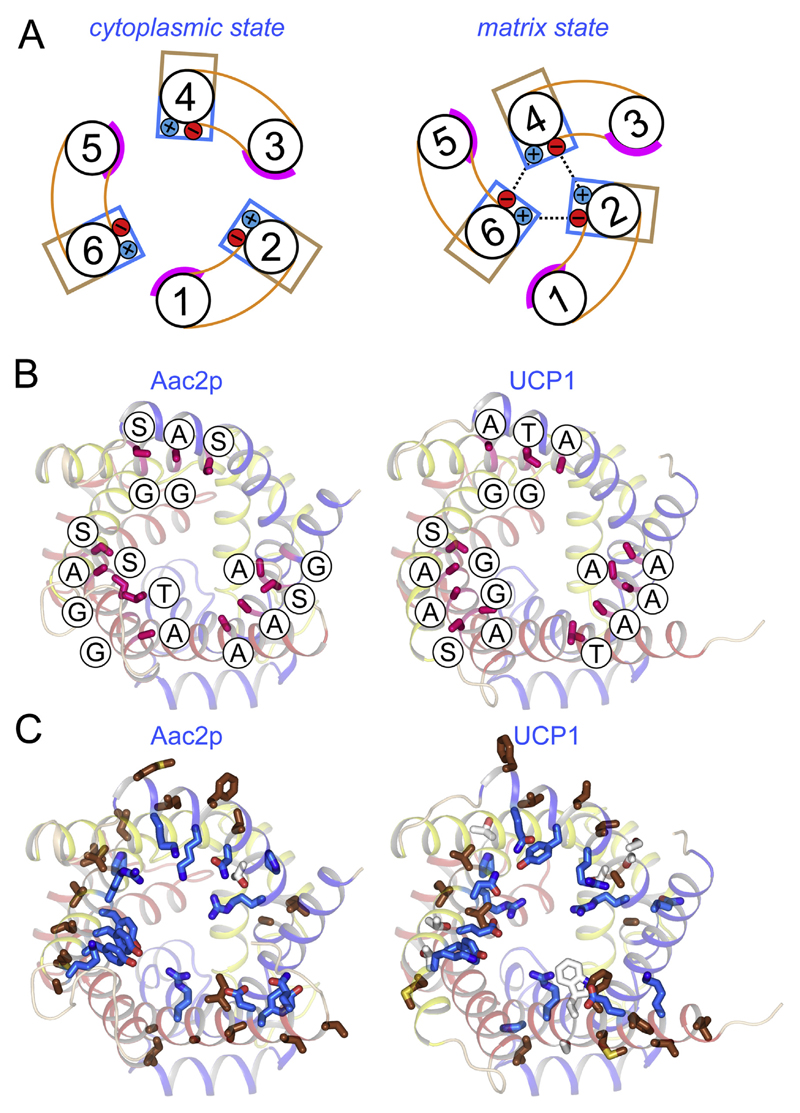
Fig. 9. Uncoupling protein 1 has retained residues consistent with a dynamic inter-domain interface.
A) Schematic representation of the α-helical arrangement of mitochondrial carriers in the cytoplasmic (left) and matrix state (right), caused by rotation of the three domains [105]. The smooth surfaces of the odd numbered helices are shown in magenta, whereas the hydrophilic and hydrophobic residues of the even-numbered α-helices are represented by blue and brown boxes, respectively. The formation of the cytoplasmic salt bridge network is also shown. B) and C) The structures of the ADP/ATP carrier (left) and uncoupling protein 1 (right), viewed from the cytoplasmic side. Domain 1, 2 and 3 are coloured blue, yellow, and red, respectively. B) Residues of the odd-numbered α-helices in the inter-domain interface have no or small side chains, including the residues of the conserved GXXXG motif (shown in magenta and labelled). (C) Residues of the even-numbered α-helices in the inter-domain interface are hydrophobic (brown), facing the membrane, or hydrophilic (blue), facing the cavity.