Central nervous system (CNS) infiltration is rarely detected at initial diagnosis of pediatric acute lymphoblastic leukemia (ALL). This is mainly due to a lack of sensitivity of cerebrospinal fluid (CSF) diagnostics by cytology. Currently, all patients receive extensive CNS-directed chemotherapy regardless of the presence of leukemic cells in the CSF, which is associated with a number of neurological toxicities. Some high-risk patients also receive cranial irradiation, which carries an increased risk for secondary malignancies. On the other hand, omitting this type of CNS-directed therapy will lead to CNS relapses in a vast majority of the patients1 suggesting that CNS tropism is an omnipresent feature in ALL blasts. Even though the prognosis of pediatric ALL has been continuously improving on contemporary treatment protocols, the CNS compartment is affected in roughly one-third of ALL relapses.2 Importantly, CNS involvement at relapse occurs mainly in patients who were CNS negative at initial diagnosis.3 This suggests that ALL cells that are refractory to therapy or particularly receptive to microenvironment-derived protective signals are able to survive in the CNS niche for prolonged periods of time as extramedullary minimal residual disease. In order to solve the mechanistic problem of CNS disease in ALL, factors influencing the homing and the survival of leukemic cells in this protective sanctuary are increasingly being investigated.
In this issue of Haematologica, Gaynes et al. report a set of CNS-related genes identified by a screening approach in NALM-6 B-cell precursor (BCP)-ALL cells recovered from the bone marrow and CNS compartments of NOD/SCID-gamma (NSG) mice.4 They further investigated the role of the homeobox gene PBX1, one of the genes up-regulated in the CNS by using BCP-ALL cell lines in a co-culture model of the blood brain barrier (BBB) and in xenografts. They found PBX1 protein levels to be up-regulated in different BCP-ALL cell lines in co-culture with murine choroid plexus cells and in the CNS of xenografts. In co-culture, PBX1 conferred chemotherapy resistance to cytarabine and methotrexate, both of which are important drugs for CNS-directed therapy in ALL. Targeting PBX1 by RNA interference decreased the protective effects induced in this co-culture model pointing at a role of PBX1 as a niche-specific survival factor. As expected, ectopic expression of PBX1 enhanced the colony-forming ability of ALL cell lines in vitro and, importantly, CNS infiltration in vivo.
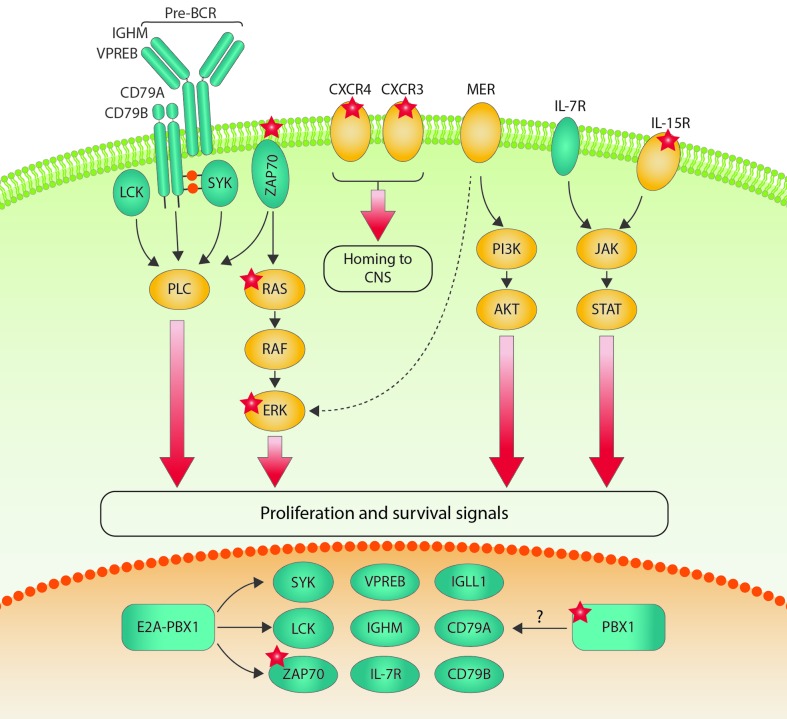
PBX1 is known to support long-term self-renewal abilities in hematopoietic stem cells and, as a translocation partner for E2A in t(1;19) positive BCP-ALL, it leads to an arrest of B cells at the pro-/pre-B-stage and is also able to enhance self-renewal capacity in pre-leukemic B-cell progenitors.5 It has been previously shown that enforced expression of PBX1 has no transforming potential in NIH3T3 cells.6 In contrast, the oncogenic potential of the E2A-PBX1 fusion has been demonstrated in a variety of experimental models.5,7 Interestingly, E2A-PBX1 rearranged BCP-ALL has a particular propensity to enter the CNS.8 The E2A-PBX1 fusion includes almost the entire coding sequence of PBX1, is predominantly localized in the nucleus, and acts as a constitutive transcriptional activator.9 E2A-PBX1 binds a subset of targets also bound by PBX1, raising the possibility that both regulate similar genes, which may also be relevant for CNS disease in ALL. The TAM-receptor MER is over-expressed in E2A-PBX1 rearranged BCP-ALL and has been found to mediate a quiescent and chemo-resistant phenotype in leukemic cells in the CNS of pediatric t(1;19) positive patients.10 There is, however, no evidence for a direct regulation of MER by either E2A-PBX1 or PBX1. In contrast, E2A-PBX1 was found to induce pre-B-cell receptor (pre-BCR) signaling by activating the transcription of the pre-BCR kinases SYK, LCK and ZAP70 in a subset of murine leukemias in a transgenic E2A-PBX1 BCP-ALL mouse model5,11 and in human BCP-ALL.12 The E2A-PBX1 transgene caused the acquisition of a number of secondary activating mutations, most notably in the JAK/STAT and RAS pathways, suggesting that E2A-PBX1 induces genomic instability.5 Interestingly, activated JAK/STAT signaling and constitutive activation of the RAF/RAS/MEK/ERK pathway may be distinguishing features of certain leukemias invading the CNS: interleukin-15, shown to be predictive of CNS involvement and relapse in patients,13 induces both ERK1/2 and STAT5, thereby up-regulating leukocyte trafficking molecules.14 Activating mutations in the RAS pathway have been found to be highly prevalent in relapsing childhood ALL and led to CNS infiltration in xenografts which could be abrogated by MEK1/2 inhibition.15 Recently, ZAP70 kinase has been associated with CNS disease in ALL, in part via ERK1/2-mediated activation of the chemokine receptors CCR7 and CXCR4.16 A summary of the signaling pathways induced in E2A-PBX1 positive BCP-ALL and their potential role in CNS leukemia is presented in Figure 1. Whether CNS leukemogenesis by PBX1 without the E2A-PBX1 rearrangement is based on similar molecular mechanisms remains to be investigated.
Figure 1.
Surface receptors and signaling pathways involved in central nervous system (CNS) leukemia. E2A-PBX1 transcriptional targets are depicted in green. Many of the E2A-PBX1 target genes are important for pre-BCR signaling, which has also been connected to CNS leukemia. Molecules for which there is direct evidence for a role in CNS leukemia are illustrated with red stars. The overlap between genes regulated by E2A-PBX1 and by PBX1 alone remains unclear.
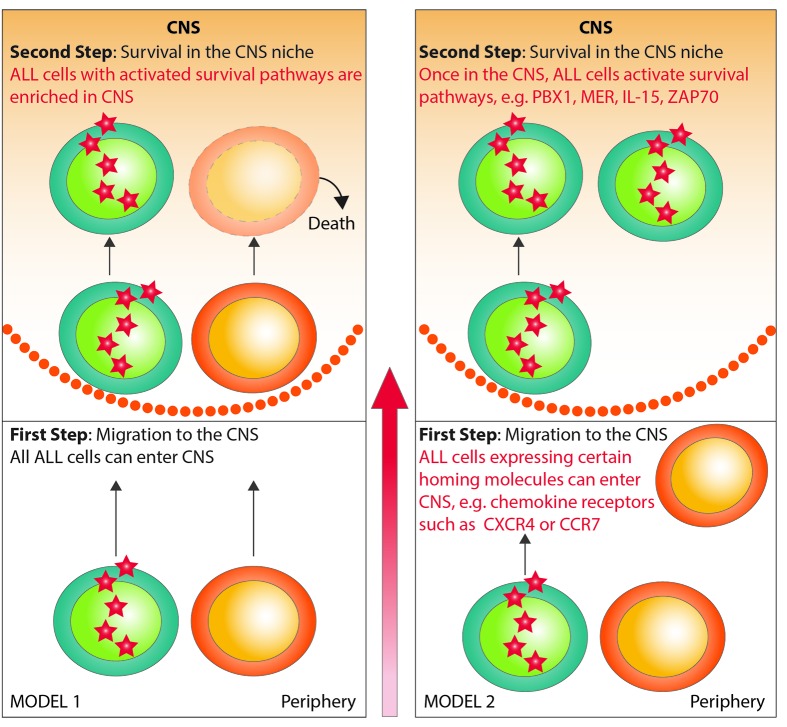
It has recently been suggested that CNS infiltration is a universal feature of leukemic blasts based on pre-clinical experimental data.17 While it is highly possible that the majority of ALL blasts are able to cross the BBB and home to the CNS, pre-clinical and clinical data suggest that ALL sub-clones expressing niche-specific survival factors may be more relevant from a mechanistic point of view as these cells may be quiescent and chemotherapy-resistant (Figure 2, model 1). On the other hand, it is also possible that only ALL cells expressing specific homing markers can enter the CNS compartment and that they activate pro-survival signaling in that niche in a second step (Figure 2, model 2). Whether PBX1-mediated CNS leukemia reflects the first or the latter mechanism remains to be elucidated using further in vivo modeling, primary patient samples, and clinical cohorts.
Figure 2.
Models of central nervous system (CNS) leukemia. In model 1, every acute lymphoblastic leukemia (ALL) cell has the ability to migrate to the CNS, but only cells with up-regulated survival pathways cause CNS leukemia. In model 2, ALL cells with up-regulated homing markers (eg. chemokine receptors) can migrate to the CNS. In a second step, they activate the survival pathways necessary to maintain CNS disease. It is essential to clarify which model is true, as this has important implications for CNS-directed prophylaxis and therapy in ALL.
The work of Gaynes et al., together with our own work and that of others, illustrates a number of challenges in the field of CNS leukemia research.
First, there have been attempts to identify target genes in blasts directly isolated from patient CSF which could confirm, among others, JAK/STAT pathway18,19 as well as ERK and BCR activation.19 Due to limited availability of material and restricted cell integrity in primary patient CSF, model systems will increasingly be needed. Co-culture models of the BBB naturally do not reflect the complexity of its composition, and the leukemic BBB may be entirely different from a healthy one. Clearly, immunodeficiency of xenograft mice can affect the modeling of CNS leukemia20 and high degrees of immunodeficiency may favor heavy ALL infiltration in all target organs, including the CNS. Also, modeling of the leukemic CNS compartment in xenografts may have to take place during chemotherapy as it remains completely unclear at which time point before, during or after therapy initiation CNS infiltration occurs in patients.
Second, the functions of novel biomarkers indicating CNS leukemia are sometimes not easy to distinguish. It has been shown that chemokine receptors are responsible for the homing of mainly T-ALL cells into the CNS,16,21 and this is also possible, although a lot less clear, in BCP-ALL. In fact, similarly to the situation for PBX1 in the current study, it is often obscure as to whether a parameter influences the homing and/or the survival of ALL cells in the CNS niche or both.
Third, parameters identified in a pre-clinical model need to be confirmed in clinical patient series. Due to the rare detection of CNS infiltration at diagnosis, patient numbers are often too small to be able to come to any valid conclusions on a specific marker. Even patient cohorts of large study groups are biased due to selection of CNS positive patients. It is important that a CNS biomarker reliably diagnoses or excludes CNS infiltration. It must also be very clear if a novel biomarker indicates CNS infiltration at initial diagnosis or if it is predictive for CNS relapse. Both scenarios may be fundamentally different regarding their mechanism.
A fourth and final point is that diagnostic strategies for the detection of CNS infiltration need to be refined independently of novel biomarkers. Microscopic examinations of CSF samples are the conventional gold standard; however, novel techniques such as flow cytometry in the CSF and genetic methods for the detection of minimal residual disease in the CNS compartment need to be thoroughly evaluated.
Taken together this work provides important insights into the biology of CNS infiltration in ALL which is important in the context of the previous findings on CNS leukemia of the past few years. PBX1 is established as a novel mechanism of survival and chemotherapy resistance for ALL in the CNS compartment independent of the E2A-PBX1 translocation but with important implications for this entity. Work like this is fundamental in order to unravel the molecular mechanisms of CNS leukemia to ultimately identify two patient subgroups: 1) patients not needing such an intensive CNS-directed therapy, thereby avoiding significant toxicity; and 2) patients with ALL cells prone to survive in the CNS. These are patients who may benefit from intensive CNS-directed therapy and novel, more specific, approaches in the future.
Supplementary Material
Acknowledgments
DMS is supported by the Max-Eder group leader program by the Deutsche Krebshilfe e. V. and the Wilhelm-Sander Stiftung.
References
- 1.Evans AE, Gilbert ES, Zandstra R. The increasing incidence of central nervous system leukemia in children. (Children’s Cancer Study Group A). Cancer. 1970;26(2):404–409. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan S, Wade R, Moorman AV, et al. Temporal changes in the incidence and pattern of central nervous system relapses in children with acute lymphoblastic leukaemia treated on four consecutive Medical Research Council trials, 1985–2001. Leukemia. 2010;24(2):450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger B, Zimmermann M, Mann G, et al. Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol. 2003;21(2):184–188. [DOI] [PubMed] [Google Scholar]
- 4.Gaynes JS, Jonart LM, Zamora EA, Naumann JA, Gossai NP, Gordon PM. The central nervous system microenvironment influences the leukemia transcriptome and enhances leukemia chemo-resistance. Haematologica. 2016. December 29 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duque-Afonso J, Feng J, Scherer F, et al. Comparative genomics reveals multistep pathogenesis of E2A-PBX1 acute lymphoblastic leukemia. J Clin Invest. 2015;125(9):3667–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monica K, LeBrun DP, Dedera DA, Brown R, Cleary ML. Transformation properties of the E2a-Pbx1 chimeric oncoprotein: fusion with E2a is essential, but the Pbx1 homeodomain is dispensable. Mol Cell Biol. 1994;14(12):8304–8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBrun DP. E2A basic helix-loop-helix transcription factors in human leukemia. Front Biosci. 2003;8:s206–222. [DOI] [PubMed] [Google Scholar]
- 8.Jeha S, Pei D, Raimondi SC, et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia. 2009;23(8):1406–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aspland SE, Bendall HH, Murre C. The role of E2A-PBX1 in leukemogenesis. Oncogene. 2001;20(40):5708–5717. [DOI] [PubMed] [Google Scholar]
- 10.Krause S, Pfeiffer C, Strube S, et al. Mer tyrosine kinase promotes the survival of t(1;19)-positive acute lymphoblastic leukemia (ALL) in the central nervous system (CNS). Blood. 2015;125(5):820–830. [DOI] [PubMed] [Google Scholar]
- 11.Duque-Afonso J, Lin CH, Han K, et al. E2A-PBX1 Remodels Oncogenic Signaling Networks in B-cell Precursor Acute Lymphoid Leukemia. Cancer Res. 2016;76(23):6937–6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng H, Hurtz C, Lenz KB, et al. Self-enforcing feedback activation between BCL6 and pre-B cell receptor signaling defines a distinct subtype of acute lymphoblastic leukemia. Cancer Cell. 2015;27(3):409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cario G, Izraeli S, Teichert A, et al. High interleukin-15 expression characterizes childhood acute lymphoblastic leukemia with involvement of the CNS. J Clin Oncol. 2007;25(30):4813–4820. [DOI] [PubMed] [Google Scholar]
- 14.Williams MT, Yousafzai Y, Cox C, et al. Interleukin-15 enhances cellular proliferation and upregulates CNS homing molecules in pre-B acute lymphoblastic leukemia. Blood. 2014;123(20):3116–3127. [DOI] [PubMed] [Google Scholar]
- 15.Irving J, Matheson E, Minto L, et al. Ras pathway mutations are prevalent in relapsed childhood acute lymphoblastic leukemia and confer sensitivity to MEK inhibition. Blood. 2014;124(23):3420–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alsadeq A, Fedders H, Vokuhl C, et al. The role of ZAP70 kinase in acute lymphoblastic leukemia infiltration into the central nervous system. Haematologica. 2017;102(2):346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams MT, Yousafzai YM, Elder A, et al. The ability to cross the blood-cerebrospinal fluid barrier is a generic property of acute lymphoblastic leukemia blasts. Blood. 2016;127(16):1998–2006. [DOI] [PubMed] [Google Scholar]
- 18.van der Velden VH, de Launaij D, de Vries JF, et al. New cellular markers at diagnosis are associated with isolated central nervous system relapse in paediatric B-cell precursor acute lymphoblastic leukaemia. Brit J Haematol. 2016;172(5):769–781. [DOI] [PubMed] [Google Scholar]
- 19.Hicks C, Sitthi-Amorn J, Douglas J, et al. Molecular Analysis of Central Nervous System Disease Spectrum in Childhood Acute Lymphoblastic Leukemia. Clin Med Insights Oncol. 2016;10:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frishman-Levy L, Shemesh A, Bar-Sinai A, et al. Central nervous system acute lymphoblastic leukemia: role of natural killer cells. Blood. 2015;125(22):3420–3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buonamici S, Trimarchi T, Ruocco MG, et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature. 2009;459(7249):1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.