Acute kidney injury (AKI) is a leading postoperative complication and is associated with higher mortality and higher morbidity.1 Even minor postoperative creatinine increases below AKI criteria are associated with adverse outcome in both non-cardiac surgery2 and cardiac surgery patients.3 Hence, a method for the effective prevention of AKI is important and will eventually lead to the improvement of postoperative outcome. In the past, a variety of pharmacological agents have been trialed for perioperative renoprotection (e.g. fenoldepam, statins, human atrial natriuretic peptide, and nesiritide) but without conclusive evidence supporting their use.1 In this issue of Anesthesiology, Zarbock and colleagues present data on the long-term renoprotective effect of remote ischemic preconditioning (RIPC).4 The authors show that RIPC significantly reduced major adverse kidney events at 90 days after cardiac surgery in patients at high risk for AKI. The results of this follow-up analysis of the RenalRIP trial deliver strong evidence that RIPC provides additional long-term kidney protection. In the primary analysis of their trial, Zarbock and colleagues had demonstrated RIPC to deliver short-term postoperative kidney protection: RIPC significantly reduced the rate of AKI and the use of renal replacement therapy compared to no ischemic preconditioning.5 RIPC could therefore be a promising method for protecting the kidney from ischemia-reperfusion injury.
Remote ischemic preconditioning is an experimental therapeutic strategy to protect organs against the harmful effects of ischemia-reperfusion-injury by beforehand applying cycles of brief, non-detrimental ischemia and consecutive reperfusion in a distant organ (Figure 1).6 Kharbanda and colleagues were among the first to describe a non-invasive approach that was later translated to clinical use: By applying short cycles of ischemia and reperfusion to a skeletal muscle – conducted by simply inflating and deflating a standard blood pressure cuff placed on the leg – the researchers could reduce subsequently induced myocardial infarction size in pigs.7 Cheung and colleagues subsequently demonstrated the clinical application in a proof-of-concept study in humans: The authors reported that RIPC (four 5 minute cuff inflations and deflations on the thigh to 15mmHg above systolic blood pressure) prior to cardiac surgery in 37 children reduced perioperative myocardial injury by less troponin I release, lowered inotrope requirements and reduced airway pressure.8
Figure 1.
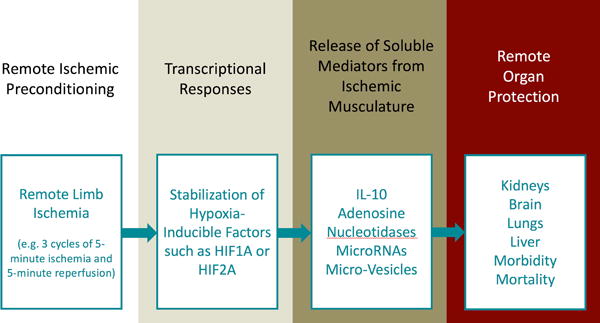
Remote Ischemic Preconditioning (RIPC): RIPC represents an experimental approach to provide organ protection. Mechanistically, short cycles of non-detrimental ischemia and reperfusion are applied to the arm or the leg. This approach is thought to drive the stabilization of transcription factors such as hypoxia inducible factors (HIF; e.g. HIF1A or HIF2A).11 This transcriptional program mediates the release of soluble mediators from the ischemic musculature into the systemic circulation. Such mediators could potentially include cytokines (e.g. IL-10), adenosine, circulating nucleotidases, microRNAs or micro-vesicles. Signalling effects of these soluble mediators on remote organs as e.g. the heart or the kidneys could than provide remote organ protection. In the current edition of Anesthesiology, Zarbock and colleagues show that RIPC provides long-term kidney protection by reducing persistent renal dysfunction and renal replacement therapy dependence in cardiac-surgery patients at high risk for acute kidney injury.4 RIPC: Remote Ischemic Preconditioning; HIF: Hypoxia-Inducible Factor; IL-10: Interleukin-10; MicroRNA: micro ribonucleic acid.
Researchers in the field have since been working on the elucidation of the underlying pathways. The stimulation with cycles of ischemia and reperfusion ultimately leads to transcriptional responses, such as the stabilization of hypoxia induced factor (HIF) HIF1A and HIF2A.9,10 These changes are than signaled to other organs via blood-borne factors in humoral pathways. Candidates to mediate distant organ protection could potentially include soluable mediators as adenosine, soluable nucleotidases, IL-10, microRNAs or micro-vesicles, leading to the activation of a protective intracellular signal transduction cascade in the target organ.6,11–13 By this means, RIPC attenuates the detrimental effects of an upcoming ischemic event in a distant organs as the heart, the lungs, the liver or the kidneys and therefore may eventually reduce not only organ injury but also morbidity and mortality.
In this issue, Zarbock and colleagues present follow-up results of their randomized controlled clinical RenalRIP trial.5 The multi-center double-blinded trial demonstrated short-term postoperative kidney protection by RIPC in patients undergoing cardiac surgery and at high risk for AKI. RIPC reduced the rate of AKI within the first 72 hours after surgery, reduced the need for renal replacement therapy and reduced the intensive care unit stay. In the current follow-up analysis, the authors show that RIPC also causes long-term kidney protection and the enhanced renal recovery of those patients who did have post-operative acute kidney injury. The authors could show that RIPC reduced the frequency of the composite endpoint major adverse kidney events (MAKE; consisting of mortality, need for renal replacement therapy, and persistent renal dysfunction) at 90 days after surgery. When analyzing the components of MAKE90, RIPC significantly reduced persistent renal dysfunction (absolute risk reduction of 13%) and renal replacement therapy dependence (absolute risk reduction 7%) at 90 days after surgery but did not influence mortality. Intriguingly, of those patients who did develop AKI within 72h after cardiac surgery, fewer suffered from persistent renal dysfunction or dialysis dependence at day 90 if they were treated with RIPC before surgery. These results provide strong evidence supporting the concept that RIPC delivers kidney protection in patients at high risk for AKI.
Despite these promising results, the evidence on RIPC and kidney protection is still inconclusive. Two additional multi-center studies by Meybohm and colleages (RIPHeart trial) as well as Hasenloy and colleagues (ERICCA trial) investigated the effect of RIPC on post-cardiac surgery outcome.14,15 While Zarbock’s study’s primary endpoint was postoperative renal function, the primary composite end-points of both the RIPHeart and ERICCA trial were focusing on postoperative cardiovascular complications and death. Interestingly, both Meybohm and colleagues’ as well as Hasenloy and colleagues’ results did not show any effect of RIPC, neither on the primary composite endpoint (as well as on any of its individual components), nor on secondary endpoints. In particular regarding post-cardiac surgery renal function, both trials did not show any renoprotective effects for RIPC (postoperative renal function was a secondary endpoint in both the RIPHeart and the ERICCA trial), contrasting the results of RenalRIP. The differences in the results may be explained by the different patient populations. The RenalRIP trial included only high risk patients while both the RIPHeart and ERICCA included low risk patients.
A likely reason for the contradicting results could be that the exact conditions for the most effective RIPC are difficult to identify in humans. Animal studies have revealed that this is in fact a challenging task. E.g., an experimental study designed to define optimal conditions for myocardial ischemic preconditioning in mice examined numerous different preconditioning regimens. Protocol optimization in this study included different cycle numbers, body temperatures, ischemia times, etc. before the authors were able to identify a regimen that reliably produced organ protection.16 Both RIPHeart and ERICCA used a sequence of four times five minutes ischemia with five minutes of reperfusion inbetween, whereas the RenalRIP protocol only used 3 times 5 minutes of ischemia with identical reperfusion intervals. It may very well be that the devil is in the detail and the optimal protocol for effective RIPC in humans has not yet been discovered. Systematic evaluation of RIPC protocols in humans are needed to find the optimal one for postoperative organ protection. Such studies could initially be done in volunteers to examine optimal release of soluble mediators, such as IL-10,11 prior to examining organ protection in patients. As the RIPC protocol of the present study by Zarbock and colleagues provided robust protection, it will also be critical to repeat their findings in larger patient populations and different surgical and patient settings.
In summary, the exciting finding of Zarbock and colleagues demonstrate for the first time that RIPC also has long-term renoprotective effects in high risk surgical patients. These impressive data are the first step towards clinical implementation of RIPC for kidney protection. However, since this was a relatively small study presenting a large effect size, the findings need to be confirmed in large-scale multi-center trials. It will be exciting to see this field further evolve with the hope that in the near future, RIPC may become a routine clinical strategy to provide kidney protection for surgical patients.
Acknowledgments
Funding:
Dr. Eltzschig is funded by the National Institute of Health (Bethesda, MD, USA) Grants R01-DK097075, R01-HL098294, POI-HL114457, R01-DK082509, R01-HL109233, R01-DK109574, R01-HL119837 and R01-HL133900.
Footnotes
Conflict of Interest
The authors are not supported by, nor maintain any financial interest in, any commercial activity that may be associated with the topic of this article.
References
- 1.Goren O, Matot I. Perioperative acute kidney injury. Br J Anaesth. 2015;115(Suppl 2):ii3–14. doi: 10.1093/bja/aev380. [DOI] [PubMed] [Google Scholar]
- 2.Kork F, Balzer F, Spies CD, Wernecke KD, Ginde AA, Jankowski J, Eltzschig HK. Minor Postoperative Increases of Creatinine Are Associated with Higher Mortality and Longer Hospital Length of Stay in Surgical Patients. Anesthesiology. 2015;123:1301–11. doi: 10.1097/ALN.0000000000000891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lassnigg A, Schmid ER, Hiesmayr M, Falk C, Druml W, Bauer P, Schmidlin D. Impact of minimal increases in serum creatinine on outcome in patients after cardiothoracic surgery: do we have to revise current definitions of acute renal failure? Crit Care Med. 2008;36:1129–37. doi: 10.1097/CCM.0b013e318169181a. [DOI] [PubMed] [Google Scholar]
- 4.Zarbock A, Kellum JA, Van Aken H, Schmidt C, Küllmar M, Rosenberger P, Martens S, Görlich D, Meersch M. Long Term effects of remote ischemic preconditioning on kidney function in heigh risk cardiac surgery patients: Follow-up results from the RenalRIP trial. Anesthesiology. 2017 doi: 10.1097/ALN.0000000000001598. [DOI] [PubMed] [Google Scholar]
- 5.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Gorlich D, Kellum JA, Meersch M, Renal RI. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–41. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 6.Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D. Remote ischemic conditioning. J Am Coll Cardiol. 2015;65:177–95. doi: 10.1016/j.jacc.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–3. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 8.Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–82. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 9.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–75. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 10.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17:1391–401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Z, Luo W, Zhan H, Semenza GL. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc Natl Acad Sci U S A. 2013;110:17462–7. doi: 10.1073/pnas.1317158110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neudecker V, Brodsky KS, Kreth S, Ginde AA, Eltzschig HK. Emerging Roles for MicroRNAs in Perioperative Medicine. Anesthesiology. 2016;124:489–506. doi: 10.1097/ALN.0000000000000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randhawa PK, Jaggi AS. Unraveling the role of adenosine in remote ischemic preconditioning-induced cardioprotection. Life Sci. 2016;155:140–6. doi: 10.1016/j.lfs.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM, Investigators ET Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N Engl J Med. 2015;373:1408–17. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 15.Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K, Collaborators RIS A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N Engl J Med. 2015;373:1397–407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 16.Eckle T, Grenz A, Kohler D, Redel A, Falk M, Rolauffs B, Osswald H, Kehl F, Eltzschig HK. Systematic evaluation of a novel model for cardiac ischemic preconditioning in mice. Am J Physiol Heart Circ Physiol. 2006;291:H2533–40. doi: 10.1152/ajpheart.00472.2006. [DOI] [PubMed] [Google Scholar]


