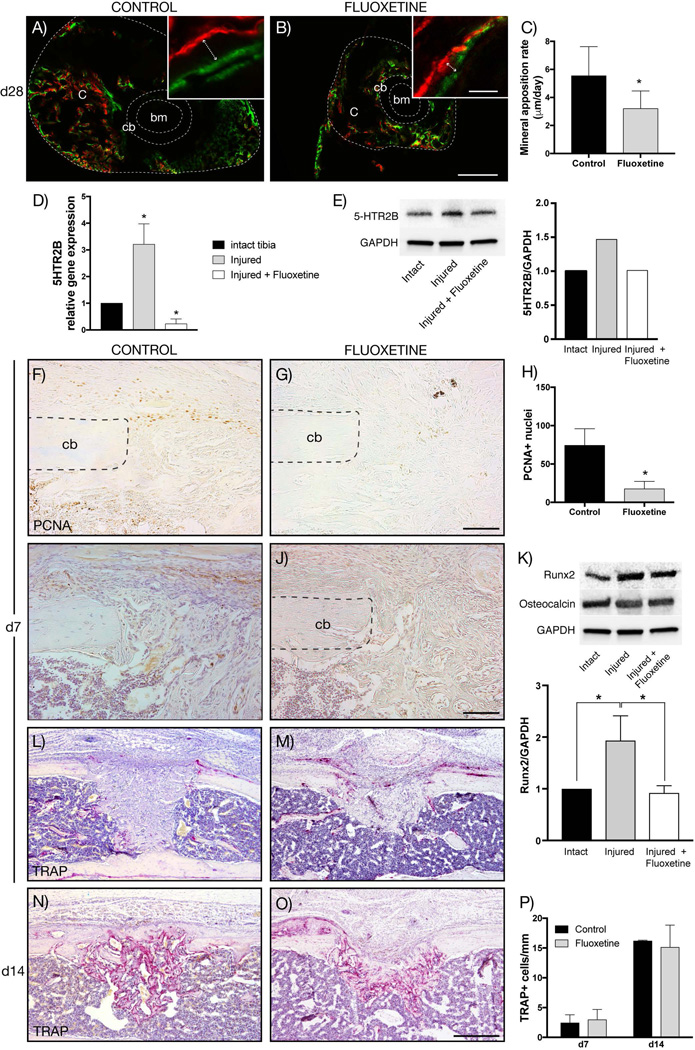
Fig. 4.
Proliferation, osteogenic differentiation, and mineralization is decreased in fluoxetine treated mice, while osteoclast activity remains unchanged. (A, B) Alizarin red (red) and calcein (green) labeling of fracture callus (outer dotted line) at POD 28. Inserts show representative high-magnification images used for quantification (arrow depicts ossification front). (C) Dynamic histomorphometry quantifying the mineral apposition rate of control and fluoxetine treated mice (n = 3, p < 0.05). (D, E) mRNA and protein expression of 5HTR2B is upregulated in response to injury at d7, while fluoxetine treatment resulted in downregulation (n = 3). (F, G) Immunohistochemistry for PCNA revealing decreased proliferation in fluoxetine-treated animals. (H) Quantification of PCNA-positive cells within the injury site (n = 4, p < 0.001). (I, J) Runx2 immunolabeling at POD 7 revealed an abundance of runx2-positive cells within the periosteum and the injury site of control animals, whereas the fluoxetine-treated animals exhibited fewer runx2-positive cells. (K) This finding was confirmed by Western blot analysis, which showed a significant decrease in Runx2 protein levels within the injury site of fluoxetine-treated animals compared to control animals. Osteocalcin levels were unchanged among groups. (L–O) Representative longitudinal section stained for TRAP in fluoxetine-treated and control animals at 7 and 14 days postsurgery. Quantification of activated osteoclasts reveals no significant differences between the two groups. Asterisk denotes statistical significance (p ≤ 0.05). Scale bar = 300 µm (A, B), 20 µm (A, B, insets), 100 µm (F, G, I, J), 500 µm (L–O). bm = bone marrow; c = callus; cb = cortical bone; d = day; oc = osteocalcin; PCNA = proliferating cell nuclear antigen; POD = postoperative day; 5HTR2B = serotonin receptor 2B.