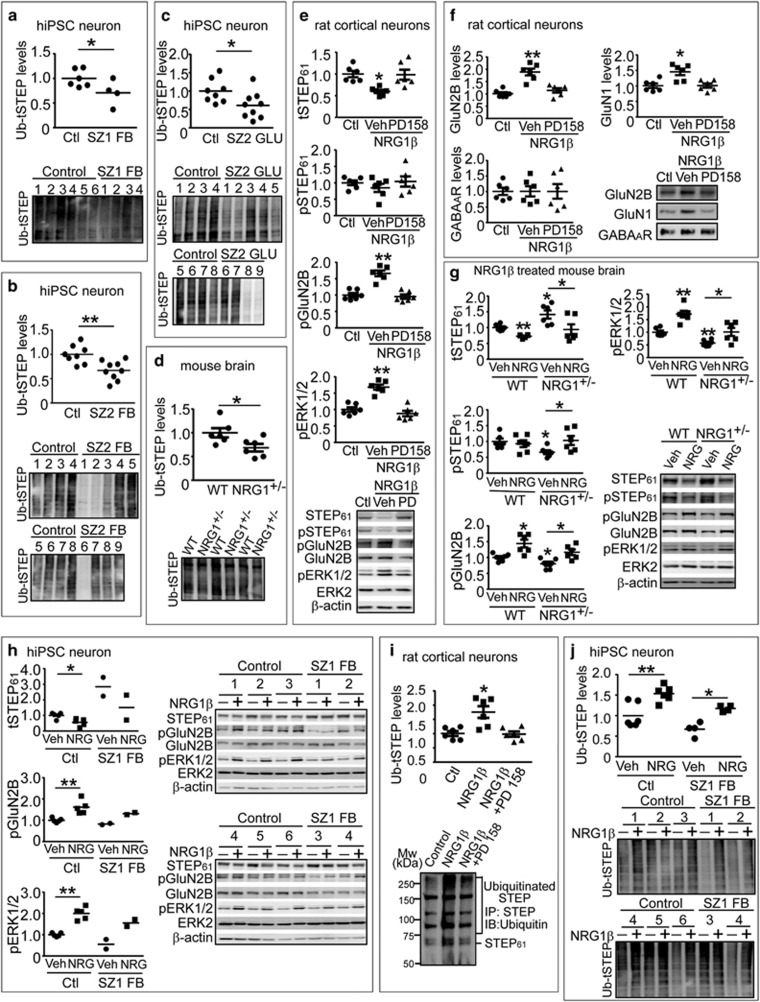
Figure 5.
NRG1 signaling leads to ubiquitination and degradation of STEP61 in rat cortical neurons and human induced pluripotent stem cell (hiPSC) neurons. (a–c) Ubiquitination of STEP is decreased in schizophrenia hiPSC neurons. Ubiquitinated protein were pulled down from SZ1-FB hiPSC neurons (a), SZ2-FB hiPSC neurons (b) or SZ2-GLU hiPSC neurons (c) using tandem ubiquitin binding entities (TUBE) agarose beads as described in Supplementary Methods, followed by detection with anti-STEP antibody. (d) Ubiquitination of STEP is decreased in Nrg1+/− mice. Male wild-type (WT) and Nrg1+/− mice (9 months old) were used to isolate ubiquitinated STEP using TUBE. (e) NRG1β treatment results in decreased STEP61 level and increased Tyr phosphorylation of GluN2B and ERK1/2 in cultures. Rat cortical neurons (14 days in vitro) were pretreated with vehicle (Veh, 0.1% dimethyl sulfoxide) or pan-ErbB receptor antagonist (PD158780, 10 μM) for 30 min, followed by NRG1β (10 nM) for 30 min. Neuronal lysates were analyzed for STEP61, pSTEP61 and tyrosine phosphorylation of STEP substrates. (f) NRG1β treatment leads to increased surface N-methyl D-aspartate glutamate receptors in cultures. Cortical neurons were treated with NRG1β (10 nM) for 30 min. Cells were incubated with 1.5 mg ml−1 Sulfo-NHS-SS-Biotin for 20 min at 4 oC, lysed in 1 × RIPA buffer. Equal amount of lysates (300 μg) was incubated with NeutrAvidin agarose to isolate biotinylated proteins, including surface receptors. Total lysates were used to measure total protein levels. (g) NRG1β administration normalizes STEP61 level and restored Tyr phosphorylation of GluN2B and ERK1/2 in Nrg1+/− mice. Male WT and Nrg1+/− littermates (6–9 months old) were administered Veh (saline) or NRG1β extracellular domain (50 ng kg−1, intraperitoneally) daily for 5 days. Frontal cortices were collected 3 h after the last injections and P2 fractions were used for biochemical analysis. (h) NRG1β treatment results in decreased STEP61 level and increased Tyr phosphorylation of GluN2B and ERK1/2 in human neurons. SZ1-FB and control neurons were treated with NRG1β (10 nM) for 6 h. STEP61 and phosphorylation of STEP substrates were assessed on western blotting. (i) NRG1 signaling leads to increased ubiquitination of STEP61 in rat cortical neurons. Cultures were pretreated with MG-132 (10 μM) for 1 h, followed by NRG1β treatment (10 nM, 30 min). All STEP species were immunoprecipitated using anti-STEP antibody and probed with anti-ubiquitin antibody. (j) NRG1β treatment leads to increased ubiquitination of STEP61 in human neurons. SZ1-FB and control neurons were pretreated with MG-132 (10 μM, 1 h), followed by NRG1β treatment (10 nM, 6 h). Ubiquitinated STEP species were pulled down using TUBE and detected by anti-STEP antibody. All data are expressed as mean±s.e.m. and statistical significance was determined using one-way analysis of variance with Bonferroni’s test (*P<0.05, **P<0.01, n=6 each group).