Abstract
Angiogenesis is implicated in diverse pathological conditions such as cancer, rheumatoid arthritis, psoriasis, atherosclerosis, and retinal neovascularization. In the present study, we investigated the effects of modified rice bran hemicellulose (MRBH), a water-soluble hemicellulose preparation from rice bran treated with shiitake enzymes, on vascular endothelial growth factor (VEGF)-induced angiogenesis in vitro and its mechanism. We found that MRBH significantly inhibited VEGF-induced tube formation in human umbilical vein endothelial cells (HUVECs) co-cultured with human dermal fibroblasts. We also observed that MRBH dose-dependently suppressed the VEGF-induced proliferation and migration of HUVECs. Furthermore, examination of the anti-angiogenic mechanism indicated that MRBH reduced not only VEGF-induced activation of VEGF receptor 2 but also of the downstream signaling proteins Akt, extracellular signal-regulated protein kinase 1/2, and p38 mitogen-activated protein kinase. These findings suggest that MRBH has in vitro anti-angiogenic effects that are partially mediated through the inhibition of VEGF signaling.
Keywords: angiogenesis, modified rice bran hemicellulose, VEGF, inhibition, signaling pathway
INTRODUCTION
Angiogenesis is the process by which new capillaries sprout from existing blood vessels [1]. It is required in several physiological processes, including development, reproduction, and wound healing [2, 3], and is also implicated in pathological conditions such as cancer, rheumatoid arthritis, psoriasis, atherosclerosis, and retinal neovascularization [2, 4,5,6,7]. Because tumor growth and metastasis are closely related to angiogenesis, angiogenesis inhibitors have been used for anticancer therapy.
The process of angiogenesis involves the proliferation, differentiation, migration, and tube formation of endothelial cells [1]. It is mainly regulated by different growth factors and their receptors [3, 8] including vascular endothelial growth factor (VEGF). Also known as VEGF-A, this dimeric glycoprotein is specific to endothelial cells and is considered one of the most important regulators of angiogenesis [9,10,11]. VEGF overexpression occurs in various tumors and contributes to tumor angiogenesis [3, 4, 11]. VEGF is also overexpressed in other pathological conditions such as rheumatoid arthritis and retinal neovascularization [3, 4, 11].
The angiogenic activity of VEGF is mediated by its binding to two tyrosine kinase receptors, VEGF receptor 1 (VEGFR1), also called Fms-like tyrosine kinase-1 (Flt-1), and VEGFR2, also called fetal liver kinase-1 (Flk-1) or kinase insert domain-containing receptor (KDR) [8, 10,11,12]. Several studies have revealed that the tyrosine kinase activity of VEGFR2 is much stronger than that of VEGFR1 and that the VEGF/VEGFR2 pathway plays a central role in angiogenic signaling [5, 13, 14]. The binding of VEGF to VEGFR2 induces dimerization and autophosphorylation of the receptor, which then leads to the phosphorylation and activation of several downstream signal transduction proteins including Akt, extracellular signal-regulated kinase (ERK)1/2, and p38 mitogen-activated protein kinase (MAPK) [8, 15]. These signaling proteins play a crucial role in regulating important cellular functions including survival, proliferation, migration, and reorganization [8, 9, 16, 17].
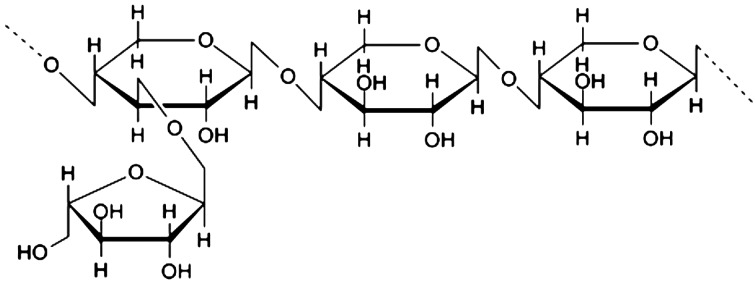
Modified rice bran hemicellulose (MRBH) is a water-soluble hemicellulose obtained by reacting rice bran hemicellulose with multiple carbohydrate hydrolyzing enzymes from shiitake mushrooms. The average molecular weight of MRBH is 8,000 Da, and the main chemical structure of MRBH is arabinoxylan, with a xylose in its main chain and an arabinose polymer in its side chain (Fig. 1) [18, 19]. Previous studies have demonstrated that MRBH has immunostimulatory effects, including enhancement of natural killer (NK) cell activity both in vitro and in vivo [20,21,22], increase of the T and B cell mitogen response [18], augmentation of macrophage phagocytosis [23], and promotion of dendritic cell (DC) maturation [24,25,26,27]. MRBH also protects against γ-irradiation-induced hematopoietic damage in mice [28] and has anti-inflammatory effects on D-galactosamine-induced hepatitis in rats [29] and chronic rheumatism [30] and irritable bowel syndrome (IBS) in humans [31]. In addition, the low molecular weight fraction (≤400 Da) of MRBH obtained by hydrolysis of MRBH with HCl at 100°C exhibited a stronger hepato-protective effect than MRBH in rats [29]. However, it remains unclear whether MRBH possesses anti-angiogenic effects. To address this possibility, we investigated the anti-angiogenic activity of MRBH in VEGF-induced angiogenesis in vitro by examining tube formation, proliferation, and migration. We also studied the signaling pathways of VEGFR2 and downstream signaling proteins Akt, ERK1/2, and p38 MAPK to elucidate the anti-angiogenic mechanism of MRBH.
Fig. 1.
Main chemical structure of MRBH.
MATERIALS AND METHODS
Reagents
Recombinant human VEGF, thiazolyl blue tetrazolium bromide (MTT), 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT), phenylmethylsulfonyl fluoride (PMSF), and 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). RIPA buffer and chemiluminescent substrate were obtained from Cell Signaling Technology, Inc. (Beverly, MA, USA). Dimethyl sulfoxide, sodium fluoride (NaF), and bovine serum albumin (BSA) were from Wako Pure Chemical Industries (Osaka, Japan). Bradford reagent was purchased from Bio-Rad Laboratories, Inc. (Hercules, CA, USA). MRBH, also called MGN-3 or BioBran, was provided by Daiwa Pharmaceutical Co., Ltd. (Tokyo, Japan).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Lonza Walkersville, Inc. (Walkersville, MD, USA), and maintained in EGM-2 endothelial cell growth medium-2 containing EBM-2 basal medium and the contents of an EGM-2 SingleQuots kit (Lonza) at 37°C and with 5% CO2.
Human dermal fibroblasts (HDF) were purchased from Cell Applications, Inc. (San Diego, CA, USA). They were maintained in Eagle’s minimum essential medium (Nissui, Tokyo, Japan) with 10% fetal bovine serum (FBS, Japan Bio Serum, Hiroshima, Japan) at 37°C and with 5% CO2.
Tube formation assay
HUVECs and HDF were co-cultured in 24-well plates according to the method of Bishop [32]. The co-cultured cells were cultivated for 10 days in EGM-2 in the presence or absence of VEGF (10 ng/ml) with or without MRBH (0.3, 1, or 3 mg/ml). The medium was refreshed every 2–3 days. At the end of cultivation, the cells were fixed with 70% ice-cold ethanol. They were incubated with a mouse anti-human platelet-endothelial cell adhesion molecule-1 (PECAM-1, a specific marker of endothelial cells highly expressed at endothelial cell intercellular junctions [33]) antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 hr at 37°C and then with a goat anti-mouse IgG alkaline phosphatase-conjugated antibody (Sigma) for 1 hr at 37°C. The cells were subsequently stained with BCIP/NBT. After five randomly selected fields of each well were photographed, the area, length, joints, and paths of the tubes were quantified using Angiogenesis Image Analyzer Ver. 2 (Kurabo, Osaka, Japan).
Cell proliferation assay
HUVEC proliferation was evaluated by estimating the viable cells using the MTT formazan production method [34]. Briefly, HUVECs (2 × 103 cells/well) were seeded in a 96-well plate in EGM-2 and incubated at 37°C with 5% CO2. After 24 hr, the medium was removed, and EBM-2 with 2% FBS was added. Cells were incubated for a further 24 hr and then were subsequently incubated in fresh medium in the presence or absence of VEGF (10 ng/ml) with or without MRBH (0.3, 1, or 3 mg/ml) for 72 hr. To assay for proliferation, 10 µl MTT reagent per well was added and further incubated for 4 hr. The formazan produced was solubilized with dimethyl sulfoxide, and the absorbance of the formazan solution was measured in a microplate reader at 550 nm.
Cell migration assay
A cell migration assay was performed using a scratch wound healing format. HUVECs were seeded onto collagen-coated 12-well plates in EGM-2 and allowed to form confluent monolayers. The cells were then washed with phosphate-buffered saline (PBS) and cultured in EBM-2 with 1% FBS for 24 hr. A scratch was created on each confluent monolayer using a 200 µl sterile pipette tip perpendicular to the bottom of the well. After washing with PBS, the cells in EBM-2 with 1% FBS were treated with vehicle (control), VEGF (10 ng/ml), or VEGF plus MRBH (0.3, 1, or 3 mg/ml) for 21 hr. The number of cells migrating into the wounded region in four randomly selected fields per well was then counted.
Western blot analysis
Subconfluent HUVECs were starved in EBM-2 with 0.3% FBS for 17 hr. After refreshing the medium, the cells were preincubated with vehicle or MRBH (3 mg/ml) for 30 min and then incubated with or without VEGF (10 ng/ml) for 15 min. To prepare whole cell lysates, cells were lysed in RIPA buffer supplemented with 1 mM AEBSF, 50 mM NaF, and 1 mM PMSF. The supernatant was obtained by centrifuging the lysate at 16,000×g for 30 min at 4°C and was used for western blot analysis. Protein concentrations were measured using the Bradford protein assay. Equal amounts of total proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes. The membranes were blocked with blocking buffer (5% BSA with 0.1% Tween-20 in Tris-buffered saline) for 1 hr at room temperature. After blocking, the membranes were incubated overnight at 4°C with one of the following primary antibodies: anti-VEGFR2, anti-phospho-VEGFR2 (Tyr1175), anti-Akt, anti-phospho-Akt (Ser473), anti-ERK1/2, anti-phospho-ERK1/2 (Thr202/Tyr204), anti-p38, anti-phospho-p38 (Thr180/Tyr182), or β-actin (Cell Signaling Technology). Subsequently, the membranes were incubated with alkaline phosphatase conjugated goat anti-rabbit IgG (Sigma) for 1 hr at room temperature. Specific bands were detected by the chemiluminescent substrate with an AE-9300 Ez-Capture MG chemiluminescent imaging system (ATTO, Tokyo, Japan) and analyzed by CS Analyzer 3.0 (ATTO).
Statistical analysis
Results are expressed as the mean ± SEM. Statistical analysis was performed using the Tukey-Kramer HSD test. A p-value less than 0.05 was considered statistically significant.
RESULTS
MRBH inhibits VEGF-induced tube formation
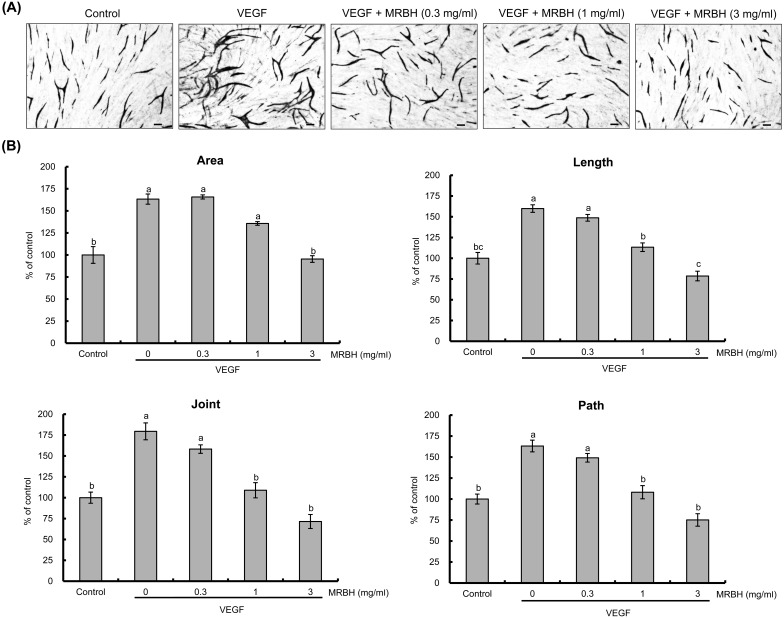
We first evaluated the anti-angiogenic effect of MRBH on VEGF-induced tube formation in HUVECs co-cultured with HDF at concentrations of 0.3, 1, and 3 mg/ml. After 10 days of co-culture, the cells were fixed and stained for PECAM-1. Representative images are shown in Fig. 2(A). Quantitative analysis indicated that MRBH dose-dependently inhibited VEGF-induced capillary-like structure formation including the area, length, joint, and path of tubes (Fig. 2(B)).
Fig. 2.
Effect of MRBH on VEGF-induced tube formation.
HUVECs co-cultured with HDF were treated with vehicle (control), VEGF (10 ng/ml), or VEGF plus MRBH (0.3, 1, or 3 mg/ml) for 10 days. After the cells were fixed and stained, the images were photographed (A), and the area, length, joints, and paths of tubes were quantified using an angiogenesis image analyzer (B). Scale bars, 100 μm. Data in B are presented as the mean ± SEM (n=4). Means not sharing a common letter are significantly different (p<0.01).
MRBH inhibits VEGF-induced cell proliferation
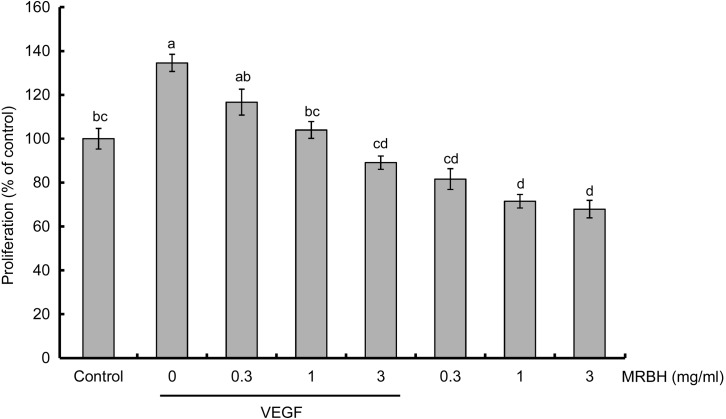
Next, we investigated the effect of MRBH on HUVEC proliferation using the MTT assay. VEGF at 10 ng/ml increased cell proliferation to 135% that of the control (Fig. 3). MRBH suppressed the VEGF-induced increase in proliferation in a concentration-dependent manner and had a significant effect at a concentration of 1 mg/ml. MRBH alone also decreased cell proliferation but to a lesser extent than in the presence of VEGF. No significant difference was observed among the three concentrations (0.3, 1, and 3 mg/ml) of MRBH alone.
Fig. 3.
Effect of MRBH on VEGF-induced cell proliferation.
HUVECs were treated with vehicle (control), VEGF (10 ng/ml), VEGF plus MRBH (0.3, 1, or 3 mg/ml), or MRBH alone (0.3, 1, or 3 mg/ml) for 72 hr. Cell proliferation was estimated by the MTT assay. Data are presented as the mean ± SEM (n=4). Means not sharing a common letter are significantly different (p<0.01).
MRBH inhibits VEGF-induced cell migration
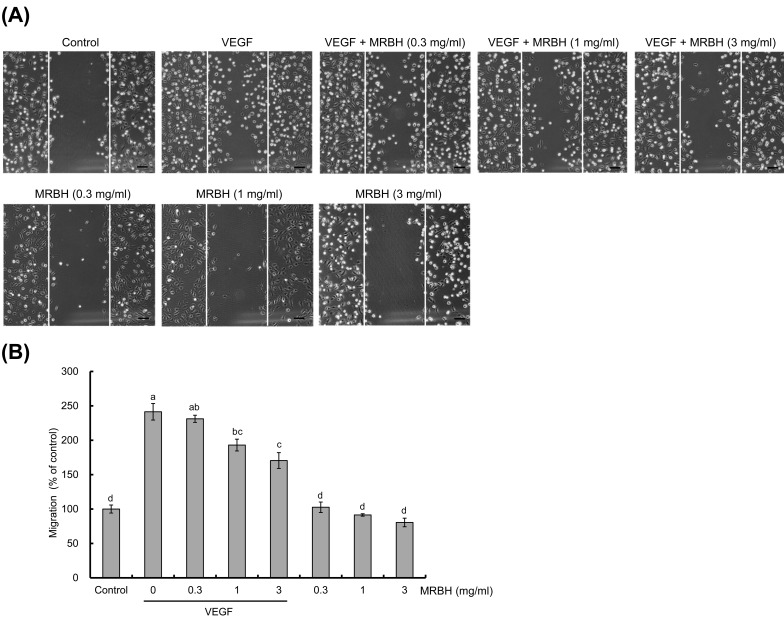
The effect of MRBH on HUVEC migration was examined in a wound-healing assay. Confluent monolayers of HUVECs on collagen-coated 12-well plates were scratch wounded and treated with vehicle (control), VEGF (10 ng/ml), VEGF plus MRBH (0.3, 1, or 3 mg/ml), or MRBH alone (0.3, 1, or 3 mg/ml) for 21 hr. Images were then taken (Fig. 4(A)), and the number of cells that migrated into the wounded region was counted (Fig. 4(B)). As shown in Fig. 4(B), migration of HUVECs in the presence of VEGF was increased to 2.4-fold that of the control. MRBH significantly inhibited this VEGF-induced migration at a concentration of 1 mg/ml, while MRBH alone had no significant effect on cell migration.
Fig. 4.
Effect of MRBH on VEGF-induced cell migration.
VEGF-induced migration of HUVECs was examined in a wound-healing assay. After the wounded HUVECs were treated with vehicle (control), VEGF (10 ng/ml), VEGF plus MRBH (0.3, 1, or 3 mg/ml), or MRBH alone (0.3, 1, or 3 mg/ml) for 21 hr, images were taken (A), and the number of cells migrating into the wounded region was counted (B). Scale bars, 100 μm. Data in B are presented as the mean ± SEM (n=4). Means not sharing a common letter are significantly different (p<0.01).
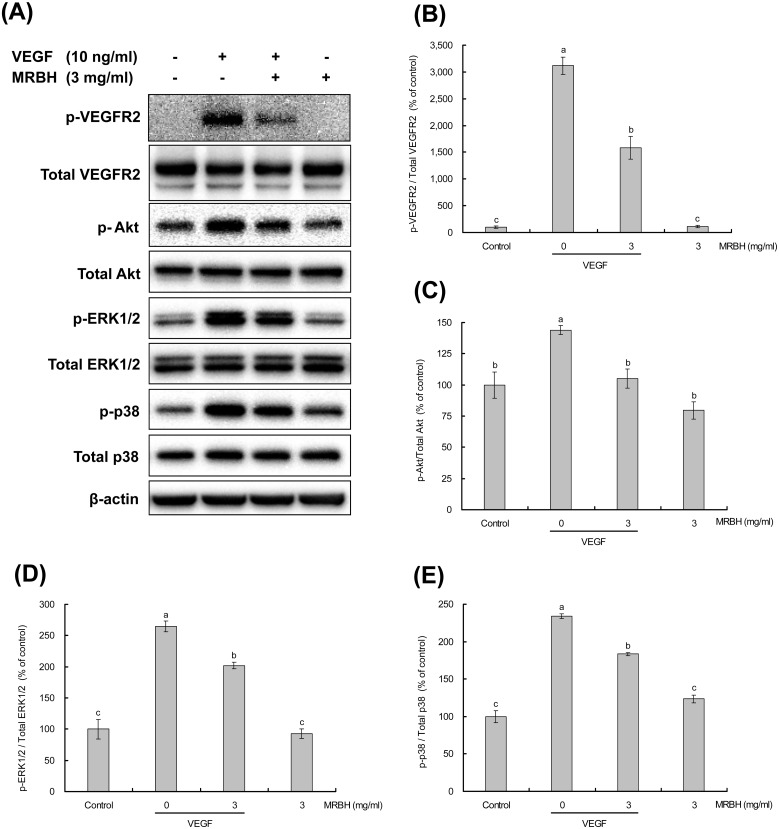
Effect of MRBH on VEGF-induced phosphorylation of VEGFR2 and downstream signaling proteins
To determine whether the anti-angiogenic effects of MRBH corresponded with the suppression of VEGFR2 and downstream signaling proteins Akt, ERK1/2, and p38, we assessed the effects of MRBH on VEGF-induced phosphorylation of these proteins in HUVECs by western blot analysis.
HUVECs were pretreated with vehicle or MRBH (3 mg/ml) for 30 min and then treated with or without VEGF (10 ng/ml) for 15 min. VEGF (10 ng/ml) treatment significantly increased the phosphorylation of VEGFR2, Akt, ERK1/2, and p38. MRBH (3 mg/ml) treatment alone caused no significant change in the expression of these proteins compared with the control. However, pretreatment with MRBH (3 mg/ml) significantly decreased the VEGF-induced phosphorylation of these proteins (Fig. 5).
Fig. 5.
Effect of MRBH on VEGF-induced phosphorylation of VEGFR2 and downstream signaling proteins.
HUVECs were pretreated with vehicle or MRBH (3 mg/ml) for 30 min and then treated with or without VEGF (10 ng/ml) for 15 min. Phosphorylation of VEGFR2, Akt, ERK1/2, and P38 in whole cell lysates was determined by western blot analysis. (A) Data are representative of three individual experiments with identical conditions. (B–E) Band intensities were quantified and represented as a bar graph. Data are presented as the mean ± SEM (n=3). Means not sharing a common letter are significantly different (p<0.05 for C and D; p<0.01 for B and E).
DISCUSSION
Angiogenesis is implicated in diverse pathological conditions including cancer and chronic inflammation such as rheumatoid arthritis and psoriasis [2, 4,5,6,7]. In this study, we found that MRBH has inhibitory effects on angiogenesis in vitro. Our results concerning anti-angiogenic mechanisms suggest that MRBH suppresses VEGF-induced activation of VEGFR2 as well as the downstream signaling proteins Akt, ERK1/2, and p38 (Fig. 5).
Earlier studies indicated that MRBH exerts anti-tumor effects in animal models [22, 35, 36] and human patients [25, 37] through the enhancement of NK activity [20,21,22, 35], an increase in the T and B cell mitogen response [18], the augmentation of macrophage phagocytosis [23], and the promotion of dendritic cell maturation [24,25,26,27]. It was also shown to have anti-inflammatory effects, with observed protective actions in acute liver injury in rats [29] and improvement effects on chronic rheumatism [30] and IBS [31] in humans.
CD95 (Fas/ APO-1) is a death receptor that belongs to the tumor necrosis factor receptor (TNFR) superfamily [38]. MRBH has been reported to sensitize leukemia cells to apoptosis mediated by the anti-CD95 antibody. MRBH treatment had no effect on the level of expression of CD95, but it caused downregulation of expression of B-cell lymphoma 2 (Bcl-2), which has been shown to protect the cells from apoptosis induced by diverse agents [39]. DEC-205 is a dendritic and epithelial cell receptor with a molecular weight of 205 kDa and belongs to the macrophage mannose receptor family of C-type lectin endocytic receptors [40]. Previous studies have demonstrated that MRBH enhances generation of cytotoxic CD8+ T cells via upregulation of DEC-205 expression on DCs [27]. MRBH also upregulates the expression of co-stimulatory molecules CD83 and CD86, which are expressed on mature DCs [26]. MRBH-stimulated DCs cause increased production of pro-inflammatory and immunoregulatory cytokines, including interleukin (IL)-1β, IL-6, IL-10, TNF-α, IL-12p40, IL-12p70, IL-2, and interferon lambda 1 (INF-λ1/IL-29/type III interferon) [26, 27]. In addition, MRBH has been shown to enhance the production of IL-12 and IFN-γ in 30 multiple myeloma patients [25]. However, it was unclear whether MRBH influences angiogenesis.
Angiogenesis is a multistep process including the proliferation, migration, and tube formation of endothelial cells [1]. The central regulator of angiogenesis is VEGF, a potent mitogen for vascular endothelial cells derived from arteries, veins, and lymphatics [3, 9, 10]. VEGF plays a prominent role in normal and abnormal angiogenesis, mainly by stimulating vascular endothelial cell proliferation, increasing vascular permeability, and promoting the survival and migration of endothelial cells [3, 8, 11].
In this study, we found that MRBH significantly inhibits VEGF-induced tube formation in HUVECs co-cultured with HDF (Fig. 2). We also observed that MRBH significantly suppresses the VEGF-induced proliferation (Fig. 3) and migration (Fig. 4) of HUVECs. These results suggest that MRBH exerts anti-angiogenic effects in vitro by inhibiting the proliferation and migration of HUVECs.
Recent studies have demonstrated that VEGF mediates its angiogenic activity mainly through the tyrosine kinase receptor VEGFR2, while the VEGF/VEGFR2 pathway is thought to be the main signaling pathway of angiogenesis [5, 13,14,15]. Survival signaling from VEGFR2 is mainly transduced through the phosphoinositide 3-kinase (PI3 K)/Akt pathway, in which Akt is recognized as a critical regulator [9, 17, 41]. Akt is considered to be a key downstream kinase in the VEGF signaling cascades because of its roles in survival, proliferation, and migration of endothelial cells [9, 17, 41, 42].
The binding of VEGF to VEGFR2 results in VEGFR2 dimerization and the autophosphorylation of the Tyr1175 residue, which is crucial for VEGF-dependent endothelial cell proliferation [43]. VEGFR2 activation leads to the activation of both ERK1/2 and p38 MAPK pathways [8, 9]. ERK1/2 plays an important role in cell proliferation [8, 15], whereas p38 MAPK is implicated in vascular permeability, actin organization, and cell migration [8, 16, 44, 45].
Our exploration of the anti-angiogenic mechanism of MRBH shows that MRBH pretreatment significantly decreased the VEGF-induced activation of VEGFR2 in HUVECs (Fig. 5(B)). This inhibition of a key receptor represents a mechanism for the suppression effects of MRBH on cell proliferation, migration, and tube formation. MRBH was reported to be partially absorbed from the gut into the blood after oral intake of MRBH in mice [46]. The results from this study suggest that MRBH might directly inhibit VEGF binding to VEGFR2 in HUVECs, leading to suppression of the VEGFR2 phosphorylation. Additionally, MRBH pretreatment significantly inhibited VEGF-induced phosphorylation of the downstream signaling proteins Akt, ERK1/2, and p38 (Fig. 5(C–E)), suggesting that the influence of MRBH on cell proliferation might be through the PI3 K/Akt and ERK1/2 pathways following VEGFR2 activation. On the other hand, the observed inhibition of VEGF-induced migration by MRBH may be partly due to the reduction of VEGF-induced p38 activation, because p38 activated by VEGFR2 is involved in cell migration [8, 15].
VEGF is upregulated in many tumors [3] and contributes to the development of solid tumors by promoting tumor angiogenesis [3, 8]. Our findings of the inhibitory effects of MRBH on VEGF-induced angiogenesis are in agreement with previous reports concerning the anti-tumor activity of MRBH [22, 25, 35,36,37]. The anti-angiogenic activity of MRBH could contribute to its tumor suppression effects, as well as its anti-inflammatory effects on humans with chronic rheumatism [30] and IBS [31] and on acute liver injury in rats [29].
In conclusion, to our knowledge, this is the first study to demonstrate that MRBH has inhibitory effects on angiogenesis in vitro. The observed inhibition of VEGFR2 and downstream signaling proteins Akt, ERK1/2, and p38 is a possible anti-angiogenic mechanism. Moreover, the effects of MRBH on tumor growth [25, 35,36,37] and inflammation [29,30,31] reported previously might also be associated with its anti-angiogenic activity. Our findings suggest that MRBH might be effective in the treatment of angiogenesis-related diseases, and this requires further study.
REFERENCES
- 1.Hanahan D, Folkman J. 1996. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86: 353–364. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Shing Y. 1992. Angiogenesis. J Biol Chem 267: 10931–10934. [PubMed] [Google Scholar]
- 3.Ferrara N. 1999. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56: 794–814. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. 1995. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1: 27–31. [DOI] [PubMed] [Google Scholar]
- 5.Shibuya M. 2008. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep 41: 278–286. [DOI] [PubMed] [Google Scholar]
- 6.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE. 1995. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 92: 905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. 1997. The codependence of angiogenesis and chronic inflammation. FASEB J 11: 457–465. [PubMed] [Google Scholar]
- 8.Takahashi H, Shibuya M. 2005. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 109: 227–241. [DOI] [PubMed] [Google Scholar]
- 9.Zachary I. 2003. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem Soc Trans 31: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 10.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. 1989. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Davis-Smyth T. 1997. The biology of vascular endothelial growth factor. Endocr Rev 18: 4–25. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Henzel WJ. 1989. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun 161: 851–858. [DOI] [PubMed] [Google Scholar]
- 13.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. 1994. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem 269: 26988–26995. [PubMed] [Google Scholar]
- 14.Seetharam L, Gotoh N, Maru Y, Neufeld G, Yamaguchi S, Shibuya M. 1995. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene 10: 135–147. [PubMed] [Google Scholar]
- 15.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. 2006. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol 7: 359–371. [DOI] [PubMed] [Google Scholar]
- 16.Rousseau S, Houle F, Landry J, Huot J. 1997. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene 15: 2169–2177. [DOI] [PubMed] [Google Scholar]
- 17.Fujio Y, Walsh K. 1999. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem 274: 16349–16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghoneum M. 1998. Anti-HIV activity in vitro of MGN-3, an activated arabinoxylane from rice bran. Biochem Biophys Res Commun 243: 25–29. [DOI] [PubMed] [Google Scholar]
- 19.Ghoneum M. 2016. From bench to bedside: the growing use of arabinoxylan rice bran (MGN-3/Biobran) in cancer immunotherapy. Austin Immunol 1: id1006, 8 pages. [Google Scholar]
- 20.Ghoneum M. 1998. Enhancement of human natural killer cell activity by modified arabinoxylane from rice bran (MGN-3). Int J Immunother 14: 89–99. [Google Scholar]
- 21.Ghoneum M, Abedi S. 2004. Enhancement of natural killer cell activity of aged mice by modified arabinoxylan rice bran (MGN-3/Biobran). J Pharm Pharmacol 56: 1581–1588. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Martínez A, Valentín J, Fernández L, Hernández-Jiménez E, López-Collazo E, Zerbes P, Schwörer E, Nuñéz F, Martín IG, Sallis H, Díaz MÁ, Handgretinger R, Pfeiffer MM. 2015. Arabinoxylan rice bran (MGN-3/Biobran) enhances natural killer cell-mediated cytotoxicity against neuroblastoma in vitro and in vivo. Cytotherapy 17: 601–612. [DOI] [PubMed] [Google Scholar]
- 23.Ghoneum M, Matsuura M. 2004. Augmentation of macrophage phagocytosis by modified arabinoxylan rice bran (MGN-3/biobran). Int J Immunopathol Pharmacol 17: 283–292. [DOI] [PubMed] [Google Scholar]
- 24.Cholujova D, Jakubikova J, Sedlak J. 2009. BioBran-augmented maturation of human monocyte-derived dendritic cells. Neoplasma 56: 89–95. [DOI] [PubMed] [Google Scholar]
- 25.Cholujova D, Jakubikova J, Czako B, Martisova M, Hunakova L, Duraj J, Mistrik M, Sedlak J. 2013. MGN-3 arabinoxylan rice bran modulates innate immunity in multiple myeloma patients. Cancer Immunol Immunother 62: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghoneum M, Agrawal S. 2011. Activation of human monocyte-derived dendritic cells in vitro by the biological response modifier arabinoxylan rice bran (MGN-3/Biobran). Int J Immunopathol Pharmacol 24: 941–948. [DOI] [PubMed] [Google Scholar]
- 27.Ghoneum M, Agrawal S. 2014. Mgn-3/biobran enhances generation of cytotoxic CD8+ T cells via upregulation of dec-205 expression on dendritic cells. Int J Immunopathol Pharmacol 27: 523–530. [DOI] [PubMed] [Google Scholar]
- 28.Ghoneum M, Badr El-Din NK, Abdel Fattah SM, Tolentino L. 2013. Arabinoxylan rice bran (MGN-3/Biobran) provides protection against whole-body γ-irradiation in mice via restoration of hematopoietic tissues. J Radiat Res (Tokyo) 54: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng S, Sanada H, Dohi H, Hirai S, Egashira Y. 2012. Suppressive effect of modified arabinoxylan from rice bran (MGN-3) on D-galactosamine-induced IL-18 expression and hepatitis in rats. Biosci Biotechnol Biochem 76: 942–946. [DOI] [PubMed] [Google Scholar]
- 30.Ichihashi K. 2004. Experience with administration of BioBran in patients with chronic rheumatism. Clin Pharmacol Ther 14: 459–463(in Japanese). [Google Scholar]
- 31.Kamiya T, Shikano M, Tanaka M, Ozeki K, Ebi M, Katano T, Hamano S, Nishiwaki H, Tsukamoto H, Mizoshita T, Mori Y, Kubota E, Tanida S, Kataoka H, Okuda N, Joh T. 2014. Therapeutic effects of biobran, modified arabinoxylan rice bran, in improving symptoms of diarrhea predominant or mixed type irritable bowel syndrome: a pilot, randomized controlled study. Evid Based Complement Alternat Med 2014: 828137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop ET, Bell GT, Bloor S, Broom IJ, Hendry NF, Wheatley DN. 1999. An in vitro model of angiogenesis: basic features. Angiogenesis 3: 335–344. [DOI] [PubMed] [Google Scholar]
- 33.Woodfin A, Voisin MB, Nourshargh S. 2007. PECAM-1: a multi-functional molecule in inflammation and vascular biology. Arterioscler Thromb Vasc Biol 27: 2514–2523. [DOI] [PubMed] [Google Scholar]
- 34.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47: 936–942. [PubMed] [Google Scholar]
- 35.Badr El-Din NK, Noaman E, Ghoneum M. 2008. In vivo tumor inhibitory effects of nutritional rice bran supplement MGN-3/Biobran on Ehrlich carcinoma-bearing mice. Nutr Cancer 60: 235–244. [DOI] [PubMed] [Google Scholar]
- 36.Noaman E, Badr El-Din NK, Bibars MA, Abou Mossallam AA, Ghoneum M. 2008. Antioxidant potential by arabinoxylan rice bran, MGN-3/biobran, represents a mechanism for its oncostatic effect against murine solid Ehrlich carcinoma. Cancer Lett 268: 348–359. [DOI] [PubMed] [Google Scholar]
- 37.Bang MH, Van Riep T, Thinh NT, Song H, Dung TT, Van Truong L, Van Don L, Ky TD, Pan D, Shaheen M, Ghoneum M. 2010. Arabinoxylan rice bran (MGN-3) enhances the effects of interventional therapies for the treatment of hepatocellular carcinoma: a three-year randomized clinical trial. Anticancer Res 30: 5145–5151. [PubMed] [Google Scholar]
- 38.Nagata S. 1997. Apoptosis by death factor. Cell 88: 355–365. [DOI] [PubMed] [Google Scholar]
- 39.Ghoneum M, Gollapudi S. 2003. Modified arabinoxylan rice bran (MGN-3/Biobran) sensitizes human T cell leukemia cells to death receptor (CD95)-induced apoptosis. Cancer Lett 201: 41–49. [DOI] [PubMed] [Google Scholar]
- 40.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM. 2000. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol 151: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, Ferrara N. 1998. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem 273: 30336–30343. [DOI] [PubMed] [Google Scholar]
- 42.Morales-Ruiz M, Fulton D, Sowa G, Languino LR, Fujio Y, Walsh K, Sessa WC. 2000. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res 86: 892–896. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi T, Yamaguchi S, Chida K, Shibuya M. 2001. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-γ and DNA synthesis in vascular endothelial cells. EMBO J 20: 2768–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Issbrücker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, Breier G, Drexler HC, Suttorp N, Clauss M. 2003. p38 MAP kinase—a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. FASEB J 17: 262–264. [DOI] [PubMed] [Google Scholar]
- 45.McMullen ME, Bryant PW, Glembotski CC, Vincent PA, Pumiglia KM. 2005. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J Biol Chem 280: 20995–21003. [DOI] [PubMed] [Google Scholar]
- 46.Endo Y, Kanbayashi H. 2003. Modified rice bran beneficial for weight loss of mice as a major and acute adverse effect of Cisplatin. Pharmacol Toxicol 92: 300–303. [DOI] [PubMed] [Google Scholar]