Abstract
Isothiocyanates, such as phenethyl isothiocyanate (PEITC), are formed following the consumption of cruciferous vegetables and generate reactive oxygen species (ROS) that lead to the induction of cytoprotective genes such as the UDP-glucuronosyltransferases (UGTs). The induction of ROS activates the Nrf2-Keap 1 pathway leading to the induction of genes through antioxidant response elements (AREs). UGT1A1, the sole enzyme responsible for the metabolism of bilirubin, can be induced following activation of Nrf2. When neonatal humanized UGT1 (hUGT1) mice, which exhibit severe levels of total serum bilirubin (TSB) because of a developmental delay in expression of the UGT1A1 gene, were treated with PEITC, TSB levels were reduced. Liver and intestinal UGT1A1 were induced, along with murine CYP2B10, a consensus CAR target gene. In both neonatal and adult hUGT1/Car−/− mice, PEITC was unable to induce CYP2B10. A similar result was observed following analysis of UGT1A1 expression in liver. However, TSB levels were still reduced in hUGT1/Car−/− neonatal mice because of ROS induction of intestinal UGT1A1. When oxidative stress was blocked by exposing mice to N-acetylcysteine, induction of liver UGT1A1 and CYP2B10 by PEITC was prevented. Thus, new findings in this report link an important role in CAR activation that is dependent upon oxidative stress.
The consumption of cruciferous vegetables such as broccoli, watercress, and cauliflower have been correlated through epidemiological studies with a lower incidence of cancer development1,2,3. These vegetables are abundant in glucosinolates, which are hydrolyzed by plant myrosinase and by microflora of the gastrointestinal tract to form isothiocyanates (ITCs), commonly regarded as conveying active anticarcinogenic activity4,5. The anticarcinogenic activity of ITCs has been linked to the activation of the nuclear factor erythroid 2 related factor 2 (Nrf2)-Keap1 signaling pathway6, which is followed by binding of the transcriptional factor Nrf2 to antioxidant response elements (AREs)7,8. Prominent in this induction process are the cluster of genes that encode proteins involved in limiting both endogenous and exogenous chemicals from initiating deleterious biological effects by directing their metabolism, an event that biologically inactivates the agents and in many cases, facilitates their excretion from the body. This cumulative defense system, carried out by proteins that have been classified to participate in Phase II drug metabolism, prevents chemical induced toxic/genetic mutations and limits induction of epigenetic events that might lead to cellular proliferation and tumor development9.
The UDP-glucuronosyltransferase (UGT) family of proteins is part of the Phase II cluster and participates in the detoxification and genoprotective process by converting target substrates to water soluble products by glucuronidation10,11. Once the glucuronides are formed, the soluble metabolites are transported out the cell, in many instances through high affinity transporters12,13,14, and become sequestered in the water compartments of the tissue that eventually lead to elimination. Thus, the UGTs play a key role in detoxification of many endogenous agents, such as steroids and bile acid, hundreds of therapeutically consumed drugs, and environmental toxicants that are known mutagens and carcinogens11. The substrate specificity attributed to UGTs occurs in part because of a large and diversified gene family of proteins. In human, 19 UGTs have been identified, with each being classified into the UGT1 (UGT1A1, -1A3, -1A4, -1A5, -1A6, -1A7, -1A8, -1A9 and -1A10) or UGT2 family (-A1, -A2, -A3, -B4, -B7, -B10, -B11, -B15, -B17 and -B28)15. Each of the UGT genes is dynamically regulated, as evident from unique expression patterns observed in human tissues11. When human tissues were examined to document gene expression patterns, each tissue displayed a unique complement of the UGTs, providing evidence that tight tissue specific regulation leads to diversified glucuronidation activity16,17,18,19.
Cellular and molecular events that underlie the regulation of the UGTs, especially those encoded by the human UGT1 gene family, have been studied in humanized transgenic mice that express the human UGT1 locus in a Ugt1-null background (humanized UGT1 mice)20. Developmental and tissue specific regulation of UGT1A genes has been shown to reflect similar patterns of expression as characterized in human tissues21. For example, the human UGT1A1 gene, which is developmentally delayed in newborns22, is also developmentally repressed in hUGT1 neonatal mice23. Since UGT1A1 is the sole enzyme responsible for the metabolism of serum bilirubin24, this delay in UGT1A1 expression leads to severe neonatal hyperbilirubinemia in hUGT1 mice. The UGT1A1 gene can be transcriptionally induced by a host of xenobiotic nuclear receptors, including the pregnane X receptor (PXR)25, the constitutive androstane receptor (CAR)26, the peroxisome proliferator-activated receptor alpha (PPARα)27, the liver X receptor (LXR), and the environmental sensors, Ah receptor28,29 and Nrf230. The oral or intraperitoneal administration of ligands capable of activating these receptors in neonatal hUGT1 mice leads to the induction of liver or intestinal UGT1A1 followed by dramatic reductions in total serum bilirubin (TSB) levels. Induction of UGT1A1 following activation of the Nrf2-Keap1 signaling pathway by oxidants, such as tert-butylhydroquinone, implicates a potential role for the AREs responding to oxidative stress in control of the UGT1A1 gene30.
It is generally believed that ITCs, such as phenethyl isothiocyanate (PEITC), induce UGT1A1 through activation of the Nrf2-Keap1 pathway, since the UGT1A1 gene is a known target of Nrf230. We have extended these findings by treating neonatal and adult hUGT1 mice with PEITC and have evaluated the impact of ITCs in vivo for its capability to reduce serum bilirubin in neonatal mice while inducing a genome wide array of potential target genes. We will demonstrate that along with Nrf2 response genes, such as Nqo1 being significantly induced in adult hepatic tissue, CAR dependent target genes, such as Cyp2b10, are also upregulated. In addition, evidence will be presented linking the generation of reactive oxygen species (ROS) by PEITC in neonatal intestinal tissue to activation of intestinal epithelial cell maturation, a process that was recently shown to be linked to derepression of the nuclear corepressor protein NCoR1 and induction of intestinal UGT1A131. These observations led us to examine in greater detail the potential cross-talk between the PEITC generated antioxidant response and CAR activation, and the implications of ROS on activation and induction of UGT1A1.
Materials and Methods
Chemical reagents
Phenethyl isothiocyanate (PEITC) and N-acetyl-L-cysteine (NAC) were obtained from Sigma-Aldrich (St. Louis, MO). Rabbit anti-UGT1A1 monoclonal antibody was purchased from abcam (Cambridge, UK). Mouse anti-GAPDH monoclonal antibody and rabbit anti-CYP2B9/10 antibody were obtained from Santa Cruz Biotechnology (Dallas, TX). Anti-p-p38, anti-p38, anti-mouse IgG horseradish peroxidase (HRP) conjugated antibody and anti-rabbit IgG HRP conjugated antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA).
Animals
Transgenic mice expressing the human UGT1 locus (UGT1A1*1) in a Ugt1−/− background (hUGT1)32 were developed previously20. hUGT1/Car−/− mice were constructed previously23. All animals were housed at the University of California San Diego (UCSD) Animal Care Facility and received food and water ad libitum. All animal use protocols, mouse handling, and experimental procedures were approved by the UCSD Animal Care and Use Committee (IACUC), and these protocols were conducted in accordance with federal regulations.
For neonatal studies, 10-day-old hUGT1, hUGT1/Car+/− or hUGT1/Car−/− mice were treated by oral gavage with corn oil (vehicle) or 200 mg/kg PEITC dissolved in vehicle. Blood and tissues were collected as indicated in the figures. For adult studies, vehicle or PEITC (200 mg/kg) was orally administrated to 6-week-old male mice every day for 4 days and tissues were collected on the 5th day after PEITC administration.
Administration of PEITC, arsenic and antioxidant NAC
Adult hUGT1 male mice (3 weeks old) were divided into 4 groups (n = 3–4 per group): control + vehicle group was given drinking water for 3 weeks and then administrated vehicle (corn oil) orally for 4 days; control + PEITC group was given drinking water for 3 weeks and then PEITC (200 mg/kg) was administrated orally for 4 days; N-acetyl cysteine (NAC) + vehicle group was given 40 mM NAC in drinking water for 3 weeks and then vehicle (corn oil) was administrated orally for 4 days; NAC + PEITC group was given 40 mM NAC in drinking water for 3 weeks and PEITC (200 mg/kg) was administrated once a day for 4 days by oral gavage. Tissues were isolated five days after vehicle or PEITC administration.
Neonatal hUGT1 mice (10 days old) were given an oral dose of NAC (75 mg/kg) 1 hour prior to the oral administration of arsenic (As3+) at a dose of 10 mg/kg. Forty-eight hours later TSB levels were taken and tissue was collected. For PEITC treatment, neonatal mice were given an oral dose of 200 mg/kg and TSB levels measured after 48 hours and tissues collected.
Bilirubin measurements
A few drops of blood were obtained from the submandibular vein and centrifuged at 2,000 × g for 5 min. Serum samples were immediately measured for total serum bilirubin (TSB) levels using a Unistat Bilirubinometer (Reichert, Inc.).
Reverse Transcription Quantitative-PCR
Tissue samples were homogenized in 0.5 mL TRIzol (Invitrogen, Waltham, MA) and total RNA from whole tissues was isolated using TRIzol reagents per the manufacturer’s instructions. Using iScript Reverse Transcriptase (Bio-Rad Laboratories, Hercules, CA), 1 μg of total RNA was used for the generation of cDNA in a total volume of 20 μL as outlined by the manufacturer. Following cDNA synthesis, quantitative PCR was carried out on a CFX96 qPCR system (BioRad) by using SsoAdvanced SYBR Green Supermix (BioRad). Primers were designed through the mouse primer depot (https://mouseprimerdepot.ncimci,.nih.gov/). The sequences of the primers used are listed in Table 1.
Table 1. Primer sequences used for Reverse Transcription Quantitative-PCR (RT-qPCR).
| Primer | Forward | Reverse |
|---|---|---|
| Human UGT1A1 | 5′-CCTTGCCTCATCAGAATTCCTTC-3′ | 5′-ATTGATCCCAAAGAGAAAACCAC3′- |
| Mouse Cyp2b10 | 5′-AAAGTCCCGTGGCAACTTCC-3′ | 5′-CATCCCAAAGTCTCTCATGG-3′ |
| Mouse Nqo1 | 5′-TTTAGGGTCGTCTTGGCAAC-3′ | 5′-GTCTTCTCTGAATGGGCCAG-3′ |
| Mouse GstA1 | 5′-AGCCCGTGCTTCACTACTTC-3′ | 5′-TCTTCAAACTCCACCCCTGC-3′ |
| Mouse GstA2 | 5′-GAATCAGCAGCCTCCCCAAT-3′ | 5′-TCCATCAATGCAGCCACACT-3′ |
| Mouse cyclophiline | 5′-CAGACGCCACTGTCGCTT-3′ | 5′-TGTCTTTGGAACTTTGTCTGC-3′ |
| Mouse Akp3 | 5′-CTGGAGCCCTACACCGACT-3′ | 5′-AGGCTTCTGGCGCTGTTAT-3′ |
| Mouse Sis | 5′-ACCCCTAGTCCTGGAAGGTG-3′ | 5′-CACATTTTGCCTTTGTTGGATGC-3′ |
| Mouse Krt20 | 5′-CCTGCGAATTGACAATGCTA-3′ | 5′-CCTTGGAGATCAGCTTCCAC-3′ |
| Mouse Glb1 | 5′-CACTGCTGCAACTGCTGG-3′ | 5′-ATGTATCGGAATGGCTGTCC-3′ |
| Mouse Lrp2 | 5′-CCAGGATTCTGGTGATGAGG-3′ | 5′-CGGGAACTCCATCACAAACT-3′ |
| Mouse Nox4 | 5′-TCTGGAAAACCTTCCTGCTG-3″ | 5′-CCGGCACATAGGTAAAAGGA-3′ |
Western blot analysis
Tissues (0.1 mg) were homogenized in 0.5 mL 1 × RIPA lysis buffer (EMD Millipore, Billerica, MA) supplemented with protease inhibitor cocktail (Sigma-Aldrich). Western blots were performed by using NuPAGE 4–12% BisTris-polyacrylamide gels (Invitrogen) with the protocols described by the manufacturer. Protein (20 μg) was electrophoresed at 200 V for 50 min and transferred at 30 V for 2 hours to PVDF membranes (EMD Millipore). Membranes were blocked with either 5% skim milk or 5% BSA (fraction V) at room temperature for 1 hour and incubated with primary antibodies (rabbit anti-human UGT1A1 (ab 194697), mouse anti-GAPDH (sc-47724), rabbit anti-CYP2B9/10 antibody, rabbit anti-p-p38 (cs-9211) or rabbit anti-p38 (cs-9212)) at 4 °C overnight. Membranes were washed and exposed to HRP-conjugated secondary antibodies (anti-mouse IgG or anti-rabbit IgG) for 1 hour at room temperature. Protein was detected by the ECL Plus Western blotting detection system (PerkinElmer) and was visualized by the BioRad gel documentation system. All Western blots are cropped from the full length blots that have been included in the Supplemental Material.
Statistical analysis
Statistical significances were determined by analysis of variance by using Student’s t test. P values < 0.05 were considered statistically significant, and statistically significant differences are indicated with *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Impact of PEITC in neonatal hUGT1 mice
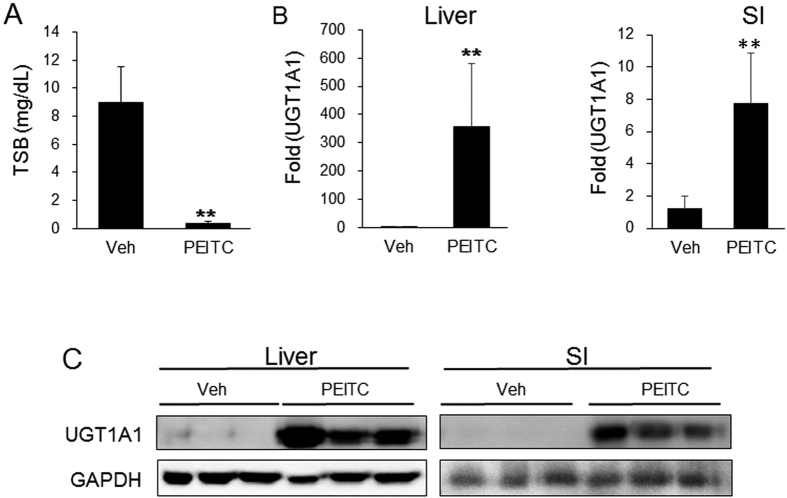
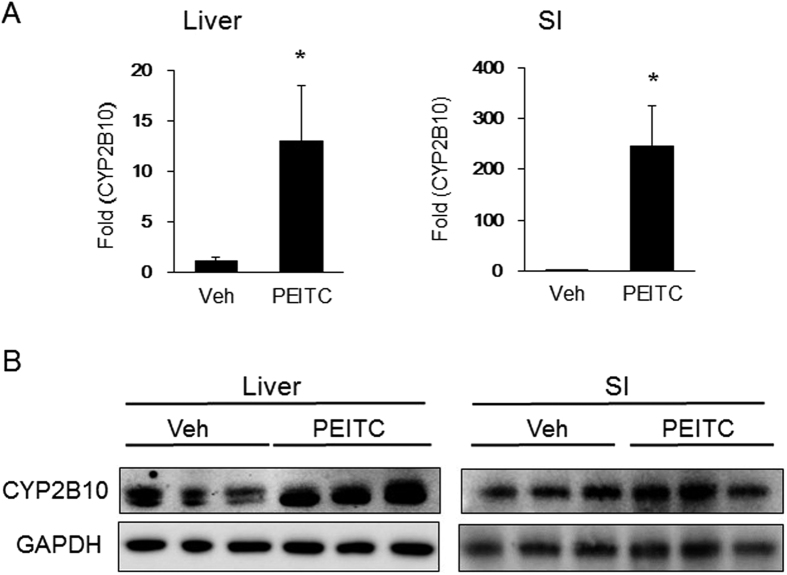
We have shown previously that xenobiotic induction of either gastrointestinal (GI) tract or liver UGT1A1 will promote the reduction of total serum bilirubin (TSB) in neonatal hUGT1 mice20,33. The impact of oral PEITC treatment to neonatal mice would be expected to induce ARE target genes both in the GI tract as absorption is occurring as well as in the liver prior to movement of PEITC into the systemic circulation. After a single oral dose of 200 mg/kg to 10-day-old hUGT1 mice, TSB levels were reduced to normal levels after 48 hours (Fig. 1A), indicating that UGT1A1 was induced. Analysis of gene expression and protein in small intestine (SI) and liver shows induction of UGT1A1 (Fig. 1B,C). Target genes of the antioxidant response, Nqo1, Gsta1 and Gsta2 were not induced dramatically in either tissue (S1), indicating that an alternative mechanism other than an antioxidant response was underlying the induction of UGT1A1. Analysis of additional potential gene expression differences that might exist between liver and SI following PEITC administration confirmed induction of the Cyp2b10 gene and protein expression in both tissues (Fig. 2), an observation that implicates a role for CAR in the induction process.
Figure 1. Induction of UGT1A1 by PEITC in neonatal hUGT1 mice.
Ten day old neonatal hUGT1 mice were treated with 200 mg/kg PEITC or vehicle by p.o. administration. (A) Forty eight hours after the treatment, total serum bilirubin (TSB) levels were taken. (B) Liver and small intestine (SI) were pulverized under liquid nitrogen and used for the preparation of total RNA or whole tissue extracts. From RNA preparations, UGT1A1 gene expression was determined by real time PCR and expressed as fold induction. (C) Using whole tissue extracts, Western blots were performed from liver and SI tissue to examine UGT1A1 expression and imaged on a BioRad ChemiDoc Touch Imaging System. The bands have been cropped from the full-length blots but not enhanced in anyway (S4). Values are the means of ±SD (n > 3). Statistically significant differences between vehicle (Veh) and PEITC are indicated by asterisks (Student t test: **P < 0.01).
Figure 2. PEITC induction of CYP2B10 in neonatal hUGT1 mice.
Following the treatment of neonatal hUGT1 mice with 200 mg/kg PEITC for 48 hours (see Fig. 1), Cyp2b10 gene and CYP2B10 protein expression were analyzed. (A) Using RNA isolated from liver and small intestines (SI), mRNA induction was quantitated by real time PCR. (B) Total tissue extracts prepared from the same tissues were used for Western blot analysis to examine CYP2B10 expression. The bands have been cropped from the full-lenth blots but not enhanced in anyway (S5). Values are the means of ±SD (n > 3). Statistically significant differences between vehicle (Veh) and PEITC are indicated by asterisks (Student t test: *P < 0.05).
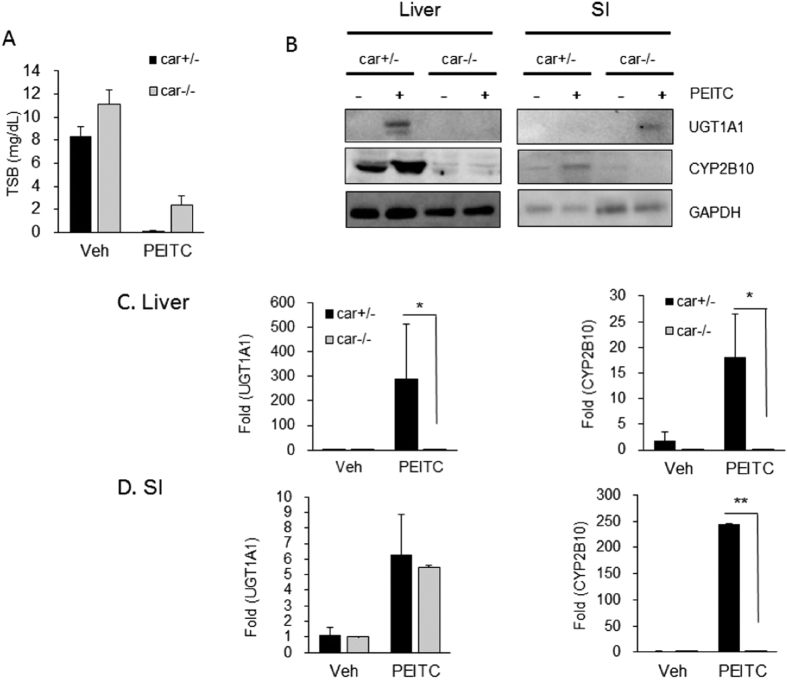
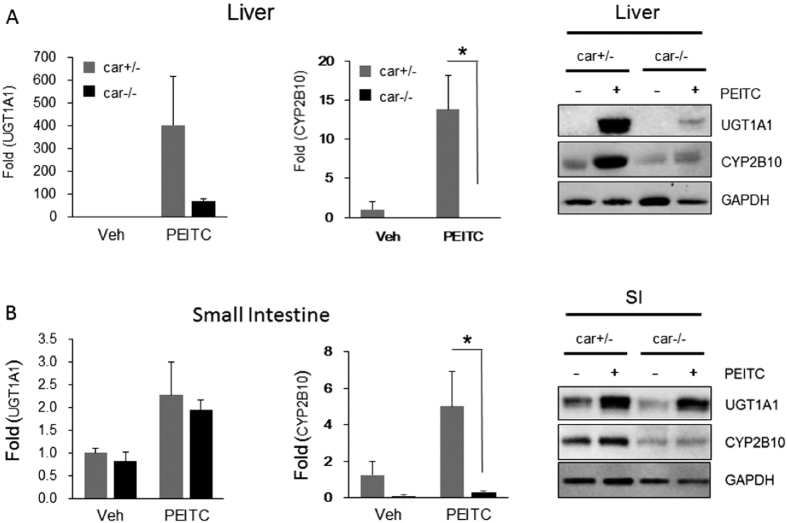
To examine the possibility that CAR is playing a role following exposure to PEITC, 10-day-old hUGT1/Car−/− mice were treated orally with PEITC and analysis of both TSB levels, UGT1A1 and CYP2B10 expression were monitored in both liver and SI (Fig. 3). TSB levels were completely reduced in hUGT1/Car+/− mice and significantly reduced in hUGT1/Car−/− litter mates but were not as low as those observed in hUGT1/Car+/− mice (Fig. 3A). Deletion of CAR led to the complete lack of PEITC initiated induction of CYP2B10 and UGT1A1 expression in liver (Fig. 3B), confirming that PEITC exposure activates liver CAR. When we examined SI, CYP2B10 expression was absent in hUGT1/Car−/− mice, but induction of UGT1A1 was observed (Fig. 3B). When gene expression patterns were examined in liver, induction of Cyp2b10 and UGT1A1 gene expression was absent in hUGT1/Car−/− mice, confirming the role of CAR in activation of these genes in hepatic tissue (Fig. 3C). Intestinal tissue showed a different induction pattern. The Cyp2b10 gene was regulated in concordance with PEITC activation of CAR, showing no activation of the gene in hUGT1/Car−/− mice. In contrast, PEITC initiated induction of UGT1A1 gene expression equally in the SI of both hUGT1/Car+/− and hUGT1/Car−/− mice (Fig. 3D), a finding that would suggest the induction of UGT1A1 in intestinal tissue is being controlled by an alternate mechanism that is not linked to CAR activation.
Figure 3. PEITC induction in hUGT1/Car-null mice.
Litters were bred to produce hUGT1/Car−/− and hUGT1/Car+/− mice and 10-day old neonatal mice treated with 200 mg/kg on day 10, followed by TSB analysis and tissue preparation on day 12. (A) After treatment, TSB levels were quantitated in hUGT/Car+/− (car+/−) and hUGT1/Car−/− (car−/−) mice. (B) Using total cell extracts from liver and small intestines (SI), Western blots were performed using antibodies toward human UGT1A1 and mouse CYP2B10. The bands have been cropped from the full-length blots but not enhanced in anyway (S6). (C) Total RNA was prepared from liver and small intestines and mRNA expression determined by real time PCR. Values are the means of ±SD (n > 3). Statistically significant differences between vehicle (Veh) and PEITC are indicated by asterisks (Student t test: *P < 0.05; **P < 0.01).
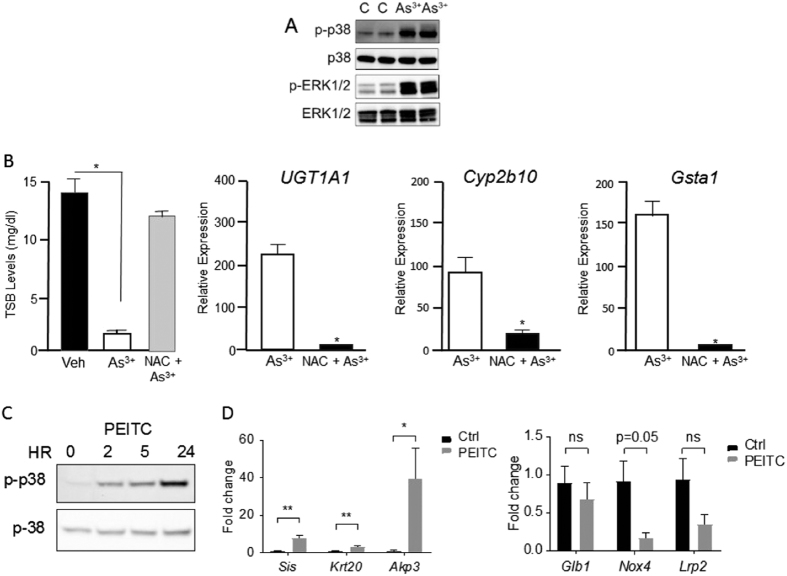
To examine this possibility in greater detail, we undertook a series of experiments to determine if an oxidative stress response could be induced in the intestines. We have shown previously that oral arsenic (As3+) exposure to neonatal hUGT1 mice induce Cyp2b10 and UGT1A1 gene expression33. If we treat neonatal hUGT1 mice with As3+ and measure phosphorylated p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase (ERK) activation, both markers of oxidative stress34, induction is observed as early as 2 hours after treatment (Fig. 4A). N-acetylcysteine (NAC), which serves as a precursor to elevate intracellular GSH35, blocks the effects of oxidative stress. We treated 10-day old hUGT1 mice with oral NAC and then challenged the mice with oral As3+, measuring gene expression after 24 hours. Arsenic treatment lead to a dramatic reduction in TSB levels, that was blocked when the mice were pretreated with NAC. Arsenic induced UGT1A1, Cyp2b10 and Gsta1 gene expression, all of which were inhibited by NAC treatment (Fig. 4B). When we measured phosphorylated p38 MAPK after PEITC treatment as an index of oxidative stress, there was a rapid and sustained activation of p38 MAPK even through 24 hours (Fig. 4C), demonstrating that PEITC elicited a strong oxidative stress response in intestinal tissue. This was also verified by an increase in GSSG in intestinal tissue (S2). However, this response was not sufficient to activate Nrf2 target genes (S1).
Figure 4. Induction of oxidative stress induces UGT1A1 in intestinal tissue.
Neonatal hUGT1 mice were given a dose of either arsenic (As3+) or PEITC and markers of oxidative stress determined. (A) Ten day old mice were given a single oral dose of As3+ (10 mg/kg) and tissue extracts from the small intestine prepared after 4 hours. Western blots were prepared and phosphorylated p38 MAPK and ERK determined. The bands have been cropped from the full-length blots but not enhanced in anyway. (B) Ten day old mice were pre-treated with 75 mg/kg N-acetylcysteine (NAC) for one hour prior to As3+ exposure. Forty-eight hours after oral As3+ exposure, RNA was isolated from small intestine and RT-qPCR was conducted for UGT1A1, Cyp2b10 and Gsta1 expression. Data is shown as fold induction of the value observed in mice treated with As3+ and water. (C) Ten-day-old mice were given an oral dose of PEITC (200 mg/kg) and intestinal tissue isolated at 0, 2, 5, and 24 hours after treatment. Western blots were performed to examine activation of phosphorylated p38 MAPK (S7). (D) Twenty-four hours after PEITC treatment, RNA was prepared from small intestines and RT-qPCR performed to examine intestinal maturation marker gene expression. Genes Sis, Akp3 and Krt20 are upregulated during maturation while genes Glb1, Nox4 and Lrp2 are downregulated (Student t test: *P < 0.05; **P < 0.01).
It has recently been demonstrated that nuclear receptor corepressor 1 (NCoR1) in intestinal epithelial tissue controls neonatal intestinal maturation by repressing gene expression31. When NCoR1 is deleted by targeted gene interruption in intestinal tissue, epithelial cell maturation is accelerated in neonatal hUGT1 mice resulting in derepression of UGT1A1 gene expression with the near complete reduction in TSB levels. Intestinal epithelial cell (IEC) maturation can be analyzed by measurement of IEC specific maturation markers, such as the upregulated Sis, Akp3, and Krt20 genes, and the downregulated Glb1, Nox4 and Lrp2 genes. Intestinal maturation can be induced by activated cellular kinases, which are believed to target phosphorylation of NCoR1 leading to derepression of gene expression36. Since PEITC is a potent inducer of intestinal p38 MAPK, we examined these maturation marker genes (Fig. 4D). Regulation of these genes followed closely the patterns of expression that were documented during neonatal intestinal maturation by expressed IKKβ and following targeted deletion of NCoR131. The possibility exists that the sharp increase in oxidative stress by PEITC promotes intestinal maturation, leading to derepression of the UGT1A1 gene and the reductions we have observed in TSB levels.
Impact of PEITC in adult hUGT1 mice
To examine the association of CAR activation and the anti-oxidative response in adult mice, we treated hUGT1/Car+/− and hUGT1/Car−/− mice orally with PEITC and evaluated gene and protein expression in liver and SI (Fig. 5). As observed in neonatal hUGT1 mice, PEITC led to induction of UGT1A1 and Cyp2b10 gene expression in liver as well as SI in hUGT1/Car+/− mice. Protein expression paralleled the findings of gene expression, with Western blots confirming induction of UGT1A1 and CYP2B10 in liver and SI. The induction of CYP2B10 in liver is completely dependent upon CAR, since no induction was noted in hUGT1/Car−/− mice (Fig. 5A). There was also a dramatic reduction in the induction of UGT1A1 in hUGT1/Car−/− mice, implicating an important role for CAR in PEITC activation. However, we did detect both at the mRNA and protein level minor induction of UGT1A1 in hUGT1/Car−/− mice, indicating that an alternative mechanism exists. Analysis of Nrf2 target genes confirmed induction was taking place by an oxidative stress response (S3). In the SI, PEITC treatment led to induction of the UGT1A1 gene and protein expression in both hUGT1/Car+/− and hUGT1/Car−/− mice (Fig. 5B). We have confirmed that PEITC does activate CAR in the SI, as shown by CAR dependent activation of the Cyp2b10 gene. While UGT1A1 is also a target following CAR activation by PEITC, the ability to induce UGT1A1 in hUGT1/Car−/− mice indicates that in this tissue the actions of PEITC are activating UGT1A1 gene expression and protein induction through an alternative signaling pathway. Since ARE genes are induced in the SI following treatment with PEITC (S3), it is reasonable to assume that an antioxidant response by PEITC is initiating induction of UGT1A1 in the SI.
Figure 5. Induction by PEITC in adult hUGT1/Car−/− mice.
Adult (8 week) hUGT1/Car−/− and hUGT1/Car+/− mice were treated by p.o administration with 200 mg/kg/day for four days. After treatment, RNA and total cell extracts were prepared. (A) From liver tissue, UGT1A1 and Cyp2b10 gene expression was analyzed by real time PCR and expressed as Fold induction. From the same tissues, total cell extracts were combined and used for Western blot analysis using anti-human UGT1A1 and anti-mouse CYP2B10 antibodies. The bands have been cropped from the full-length blots but not enhanced in anyway (S7). (B) From small intestine, a similar series of experiments were performed. Values are the means of ±SD (n > 3). Statistically significant differences between vehicle (Veh) and PEITC are indicated by asterisks (Student t test: *P < 0.05).
The role of oxidative stress and control of CAR
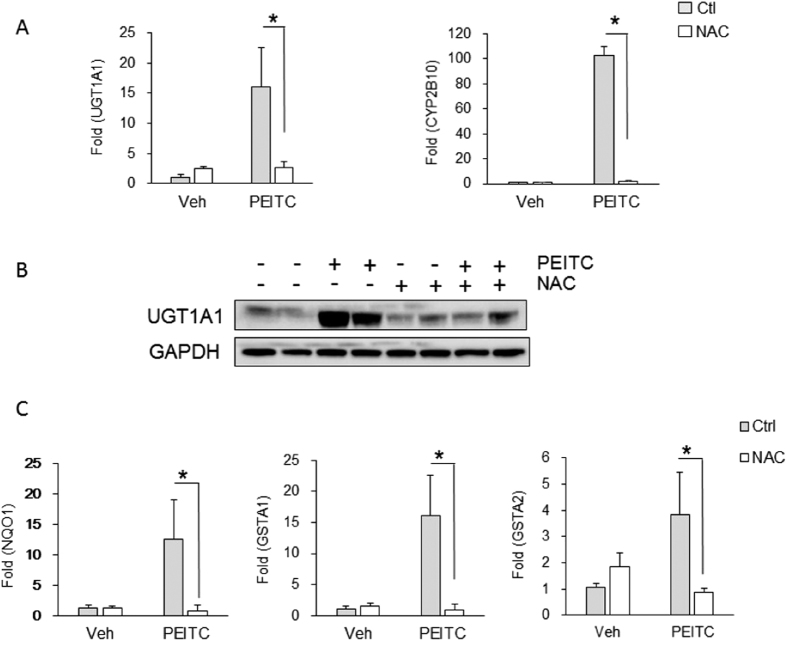
To separate an anti-oxidative response initiated by PIETC from activation of CAR and induction of UGT1A1, we first administered adult hUGT1 mice N-acetylcysteine (NAC) in their drinking water for 3-weeks. NAC prevents oxidative damage in tissues and cells by altering the redox-state and provides a reducing environment by increasing intracellular glutathione concentrations35. After two weeks of NAC exposure, hUGT1 mice were administered PIETC. Examining liver, anti-oxidant-generated gene expression patterns for Nqo1, Gsta1 and Gsta2, which were induced by PIETC, were blocked following NAC exposure (Fig. 6). Similarly, induction of gene expression for both liver UGT1A1 and Cyp2b10 by PEITC was inhibited because of NAC treatment (Fig. 6B). For UGT1A1 expression, NAC exposure completely blocked PEITC induction of UGT1A1. These results indicate that by replenishing intracellular antioxidant glutathione levels, NAC blocked PIETC-medicated antioxidant responses, and more surprisingly, abolished induction of both Cyp2b10 and UGT1A1 gene expression. These findings strongly suggesting that the Nrf2-ARE signaling pathway is actively involved in regulating these genes. Since we have demonstrated in liver that induction of the Cyp2b10 and UGT1A1 genes is driven by activation of CAR following PIETC exposure, this finding indicates that there is an important association between the production of ROS by PIETC and activation of CAR.
Figure 6. Impact of N-acetylcysteine on PEITC induction of UGT1A1.
N-acetylcysteine (NAC) was added to the drinking water at a concentration of 40 mM for 3 weeks. After NAC exposure, adult hUGT1 mice were treated by p.o. administration with 200 mg/kg PEITC once each day for four days. Liver was isolated after PEITC treatment. (A) From total RNA, human UGT1A1 and mouse Cyp2b10 gene expression was determined by real time PCR. (B) Using total cellular lysates, UGT1A1 expression was determined by Western blot analysis. The bands have been cropped from the full-length blots but not enhanced in anyway (S8). (C) Nqo1, Gsta1 and Gsta2 gene expression by real time PCR. Values are means ± SD (n = 3). Statistically significant differences between Vehicle (Veh) and PEITC are indicated by asterisks (Student t test: *P < 0.05)
Discussion
The antioxidant initiated induction patterns following exposure to PEITC have been shown to result from activation of the transcription factor Nrf2 that leads to its binding to ARE elements followed by transcriptional activation37,38,39. Activation of the Nrf2-Keap1 pathway in response to chemical and oxidative stress leads to the induction of a host of cytoprotective enzymes that provide an active defense mechanism against cellular damage. This unique signaling pathway was first discovered when ARE sequences were identified on two genes that encode enzymes involved in detoxification processes, NQO140 and GSTA241. The Nqo1 and Gsta2 genes are induced through the ARE elements following exposure to H2O2 and other agents that undergo redox cycling, or by compounds that are electrophilic. Other agents, such as the isothiocyanate PEITC, react with sulfhydryl groups to elevate the levels of ROS and reduce the antioxidant capacity, which in turn trigger the transcriptional response mediated by the AREs. Other genoprotective genes associated with cellular detoxification processes, such as UGTs, have long been implicated as important target genes that are induced following chemical and oxidative stress. A cluster of ARE elements in the phenobarbital response enhancer module (PBREM) in the UGT1A1 gene has been identified and promotes Nrf2 dependent transcriptional activation30. Based upon these findings, it would be an obvious assumption that induction of the human UGT1A1 gene following PEITC exposure would result from the production of an antioxidant response.
The previous development of hUGT1 mice offered us the opportunity to examine activation of the UGT1A1 gene since chemicals or agents administered to neonatal hUGT1 mice that result in the reduction of TSB levels are directly inducing UGT1A1 expression20,23,33,42,43. When PEITC was administered orally to neonatal hUGT1 mice, within 48 hours the TSB levels were reduced to those measured in wild type mice. Induction of UGT1A1 gene and protein expression is significant in both SI and liver, indicating that a prominent antioxidant response was initiated following PEITC dosing. However, there was no significant induction of other key antioxidant target genes related to the Nrf2 signaling pathway, such as Nqo1, Gsta1 or Gsta2, leaving us to speculate that induction of UGT1A1 was occurring independently from a Nrf2-medicated antioxidant response in the neonatal mice, although we would not rule out the possibility that Nrf2 is not fully activated at our experimental dose. Examination of additional gene expression patterns confirmed that PEITC dramatically induced Cyp2b10 gene and protein expression in both SI and liver, an important target gene that is regulated following activation of CAR. When we extended these experiments to examine the impact of oral PEITC on TSB levels and UGT1A1 expression in neonatal hUGT1/Car−/− mice, TSB levels were reduced. However, there was no induction of UGT1A1 or CYP2B10 in liver tissue, confirming that PEITC induction of the UGT1A1 and Cyp2b10 genes in neonatal hUGT1 mice is CAR dependent. While intestinal Cyp2b10 gene expression is absent in neonatal hUGT1/Car−/− mice, we still observed induction of UGT1A1 gene expression, leaving us to conclude that the actions of PIETC and induction of UGT1A1 in SI were occurring independently of CAR.
To examine this possibility, we first looked at the possibility of As3+, a known activator of oxidative stress and potent inducer of neonatal UGT1A1 and CYP2B1033, to activate stress linked proteins such as p38 MAPK. Oral As3+ rapidly activated phosphorylated p38 MAPK, demonstrating that oxidative stress was induced. When this process was blocked by pre-treatment with NAC, induction of UGT1A1 gene expression and the reduction in TSB values were blocked. This experiment indicated that activation of cellular kinases by oxidative stress may contribute to induction of intestinal UGT1A1. When we administered PEITC to neonatal hUGT1 mice, intestinal phosphorylated p-38 MAPK was dramatically activated at least through 24 hours. Recent experiments conducted in our laboratory have confirmed that derepression of NCoR1 in neonatal intestinal tissue results in activation of intestinal maturation and the induction of UGT1A131. This process may be linked to the actions of PEITC since the mechanism that leads to NCoR1 derepression requires direct phosphorylation44, an event that may be linked to PEITC activated p38 MAPK. This possibility is supported by analysis of maturation gene expression profiles, an event that we have shown leads to induction of intestinal UGT1A1 and a reduction in TSB levels31.
In adult mice, PEITC administration led to prominent induction of UGT1A1 and CYP2B10 in liver and SI tissue, but there was a clear dominance of the induction pattern in liver. In contrast to induction patterns characterized in neonatal hUGT1 mice, PEITC treatment to adult mice led to significant induction of an antioxidant response as confirmed by induction of target genes Nqo1, Gst1a and Gsta2. However, deletion of CAR in adult hUGT1/Car−/− mice resulted in the elimination of both UGT1A1 and Cyp2b10 gene induction by PEITC, confirming previous findings that induction of these genes is dependent upon CAR. Collectively, these results indicate that PEITC has dual effects on activating both CAR and Nrf2, conveying its antioxidant activity by inducing cellular detoxification processes – including the phase I Cyp2b10 and phase II, UGT1A1, Nqo1, Gsta1 and Gsta2 genes. These genes are regulated in an age- and tissue-specific fashion by PEITC with different modes of actions: Following PEITC treatment, CAR appears to be the dominant regulator in CYP2B10 induction, whereas activation of both CAR and Nrf2 is associated with UGT1A1 induction. In line with these results is the evidence that the PBREM but not the ARE region has been identified in the Cyp2b10 promoter45, although it has recently been suggested that Nrf2 is involved in phorone induction of Cyp2b1046. In contrast, both response elements have been proven to be functional and located adjacent to each other within UGT1A1 promoter sequences30. In addition, in neonates CAR has a preferential role in regulating UGT1A1, and the Nrf2-antioxidant pathway becomes more responsive to PEITC as mice reach maturity. Although liver and SI tissues generally exhibit similar induction patterns responding to PEITC exposure, our results suggest that induction of hepatic UGT1A1 is predominantly regulated by CAR, and its expression in SI may be attributed to both CAR and Nrf2.
To isolate the antioxidant response from CAR dependency in PEITC induction of UGT1A1 and CYP2B10, we exposed adult hUGT1 mice to NAC to block PEITC induced ROS production. Addition of the free radical scavenger NAC led to not only the complete inhibition of PEITC-induced expression of antioxidant genes Nqo1, Gsta1 and Gsta2 but also the blockage of CAR-initiated induction of the UGT1A1 and Cyp2b10 genes. This finding is significant because it indicates PEITC is the source of ROS enhanced expression of the UGT1A1 and Cyp2b10 genes through a ROS-dependent mechanism, further supporting the involvement of redox-sensitive transcription factor Nrf2 in controlling expression of UGT1A1 and CYP2B10. In combination with our findings that CAR is essential for PEITC initiated induction of UGT1A1, NAC blocks the PEITC induced antioxidant response, impacting induction of UGT1A1 and Cyp2b10 gene expression, suggesting an interaction between PEITC-produced oxidative stress and CAR activation, however the mechanism underlying the crosstalk between CAR and Nrf2 still needs to be determined. An outline of the proposed mechanism of PEITC initiated induction of the Cyp2b10 and UGT1A1 genes in liver and small intestine is outlined in Fig. 7.
Figure 7. The actions of PIETC and regulation of UGT1A1 gene expression.
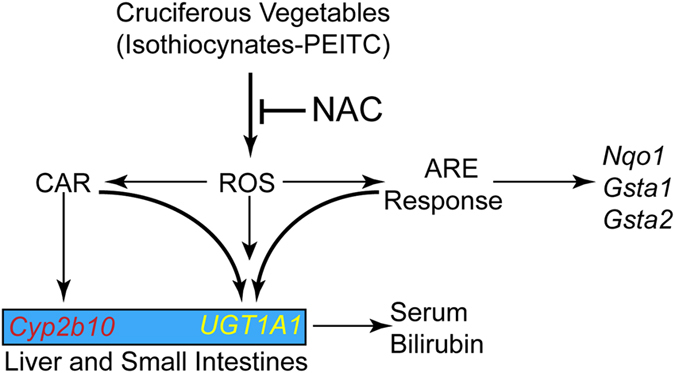
Consumption of cruciferous vegetables leads to the generation of reactive oxygen species (ROS). The ROS generates signals that leads to the activation Nrf2, facilitating an ARE response on susceptible target genes. In addition, ROS are linked to the activation of CAR in both liver and small intestines, which leads to the induction of Cyp2b10 and UGT1A1. In neonatal mice, it is also predicted that ROS have the potential to induce intestinal tissue maturation, an event that has been shown to induce the UGT1A1 gene. The induction of UGT1A1 in either the liver or small intestine will promote the metabolism and elimination of serum bilirubin. The addition of N-acetylcysteine (NAC) blocks the ROS response, nullifying both CAR and Nrf2 activation.
In adult hUGT1 mice, PEITC exposure produces an antioxidant response in both liver and SI, but the same response is not achieved in neonatal hUGT1 mice. Bilirubin is a natural antioxidant when bound to albumin47 and slightly elevated levels in individuals that inherit Gilbert’s syndrome have been documented to have reduced rates of cardiovascular disease in addition to certain cancers48. Hence, the elevated concentrations of TSB in neonatal hUGT1 mice may be sufficient to prevent PEITC induced ROS production, which is necessary to initiate an antioxidant response. When maternal mice were administered a daily dose of PEITC, nursing pups demonstrated a reduction in TSB levels (data not shown). The reduction in TSB levels correlated with induction of intestinal UGT1A1 expression. These findings indicate that neonatal hyperbilirubinemia can possibly be controlled through a maternal diet.
Our studies indicate that certain dietary ingredients present in cruciferous vegetables have a beneficial effect by providing a reductive capacity in tissues while also controlling important detoxification pathways such as glucuronidation, which are controlled at the transcriptional level through multiple signaling pathways. In addition to the important antioxidant response that leads to activation of the Nrf2-Keap1 pathway, we show here that ITCs regulate significant gene expression through a CAR dependent mechanism. However, the CAR dependent induction process, which we show exists for both the human UGT1A1 gene and the murine Cyp2b10 gene, appears to be linked mechanistically with a controlled antioxidant response. This relationship between CAR and Nrf2, which are both activated by PEITC, may serve as a double-edged-sword with regards to liver disease. Under these circumstances, agents that activate CAR stimulate the proliferative process and serve as tumor promotors49. Recently, it has been demonstrated that the accumulation of p62, a protein that can activate multiple signaling pathways, is induced in liver disease such as liver fibrosis and HCC. Induction of p62 leads to the activation of Nrf2, and sustained Nrf2 activation has been shown to be oncogenic50. Thus, in situations of liver disease, a diet rich in cruciferous vegetables would promote tumorigenesis through the activation of CAR while stimulating additional oncogenesis by activation of Nrf2. While a diet that includes cruciferous vegetables is believed to promote health by activating cellular defense systems, their consumption following liver disease may be detrimental.
Additional Information
How to cite this article: Yoda, E. et al. Isothiocyanates induce UGT1A1 in humanized UGT1 mice in a CAR dependent fashion that is highly dependent upon oxidative stress. Sci. Rep. 7, 46489; doi: 10.1038/srep46489 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was funded in part by U.S. Public Health Service Grants ES010337, GM100481, GM086713 (R.H.T.) and R21-ES024818 (S.C). We thank Mr. Ryo Mitsugi (Department of Pharmaceutics, School of Pharmacy, Kitasato University) for his technical assistance.
Footnotes
The authors declare no competing financial interests.
Author Contributions Conceptualization: E.Y, R.F. and R.H.T.; Investigation. E.Y., M.P., C.K., and S.C.; Formal analysis. E.Y. and R.H.T. Writing-Reviewing and editing, all authors. Funding acquisition; R.H.T. and S.C.
References
- Keck A. S. & Finley J. W. Cruciferous vegetables: cancer protective mechanisms of glucosinolate hydrolysis products and selenium. Integr Cancer Ther 3, 5–12 (2004). [DOI] [PubMed] [Google Scholar]
- Talalay P. & Fahey J. W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr 131, 3027S–3033S (2001). [DOI] [PubMed] [Google Scholar]
- Murillo G. & Mehta R. G. Cruciferous vegetables and cancer prevention. Nutr Cancer 41, 17–28 (2001). [DOI] [PubMed] [Google Scholar]
- Shapiro T. A. et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer 55, 53–62 (2006). [DOI] [PubMed] [Google Scholar]
- Shapiro T. A., Fahey J. W., Wade K. L., Stephenson K. K. & Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev 10, 501–508 (2001). [PubMed] [Google Scholar]
- Li W. & Kong A. N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol Carcinog 48, 91–104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau L. V. & Pickett C. B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J. Biol. Chem 266, 4556–4561 (1991). [PubMed] [Google Scholar]
- Rushmore T. H., Morton M. R. & Pickett C. B. The antioxidant responsive element Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem 266, 11632–11639 (1991). [PubMed] [Google Scholar]
- Shen G. & Kong A. N. Nrf2 plays an important role in coordinated regulation of Phase II drug metabolism enzymes and Phase III drug transporters. Biopharm. Drug Dispos 30, 345–355 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton G. J. Glucuronidation of drugs and other compounds (CRC Press, Inc., Boca Raton, 1980). [Google Scholar]
- Tukey R. H. & Strassburg C. P. Human UDP-Glucuronosyltransferases: Metabolism, Expression, and Disease. Annu Rev Pharmacol Toxicol 40, 581–616 (2000). [DOI] [PubMed] [Google Scholar]
- Keppler D., Leier I., Jedlitschky G. & Konig J. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance protein MRP1 and its apical isoform MRP2. Chem. Biol. Interact 111–112, 153–161 (1998). [DOI] [PubMed] [Google Scholar]
- Kamisako T. et al. Transport of monoglucuronosyl and bisglucuronosyl bilirubin by recombinant human and rat multidrug resistance protein 2. Hepatology 30, 485–490 (1999). [DOI] [PubMed] [Google Scholar]
- Keppler D. & Konig J. Hepatic secretion of conjugated drugs and endogenous substances. Semin. Liver Dis 20, 265–272 (2000). [DOI] [PubMed] [Google Scholar]
- Mackenzie P. I. et al. Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet. Genomics 15, 677–685 (2005). [DOI] [PubMed] [Google Scholar]
- Strassburg C. P., Manns M. P. & Tukey R. H. Differential down regulation of the UDP-glucuronosyltransferase 1A locus is an early event in human liver and biliary cancer. Cancer Res 57, 2979–2985 (1997). [PubMed] [Google Scholar]
- Strassburg C. P., Oldhafer K., Manns M. P. & Tukey R. H. Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol. Pharmacol 52, 212–220 (1997). [DOI] [PubMed] [Google Scholar]
- Strassburg C. P., Nguyen N., Manns M. P. & Tukey R. H. Polymorphic expression of the UDP-glucuronosyltransferase UGT1A gene locus in human gastric epithelium. Molecular Pharmacology 54, 647–654 (1998). [PubMed] [Google Scholar]
- Strassburg C. P., Manns M. P. & Tukey R. H. Expression of the UDP-glucuronosyltransferase 1A locus in human colon. Identification and characterization of the novel extrahepatic UGT1A8. J. Biol. Chem 273, 8719–8726 (1998). [DOI] [PubMed] [Google Scholar]
- Fujiwara R., Nguyen N., Chen S. & Tukey R. H. Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc. Natl. Acad. Sci. USA 107, 5024–5029 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. et al. Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J. Biol. Chem 280, 37547–37557 (2005). [DOI] [PubMed] [Google Scholar]
- Leakey J. E., Hume R. & Burchell B. Development of multiple activities of UDP-glucuronyltransferase in human liver. Biochem J 243, 859–861 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara R., Chen S., Karin M. & Tukey R. H. Reduced expression of UGT1A1 in intestines of humanized UGT1 mice via inactivation of NF-kappaB leads to hyperbilirubinemia. Gastroenterology 142, 109–118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma P. J. et al. Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J. Biol. Chem 269, 17960–17964 (1994). [PubMed] [Google Scholar]
- Xie W. et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA 100, 4150–4155 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani J. et al. Transcriptional regulation of human UGT1A1 gene expression: activated glucocorticoid receptor enhances constitutive androstane receptor/pregnane X receptor-mediated UDP-glucuronosyltransferase 1A1 regulation with glucocorticoid receptor-interacting protein 1. Mol. Pharmacol 67, 845–855 (2005). [DOI] [PubMed] [Google Scholar]
- Senekeo-Effenberger K. et al. Expression of the Human UGT1 Locus in Transgenic Mice by 4-Chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic Acid (WY-14643) and Implications on Drug Metabolism through Peroxisome Proliferator-Activated Receptor a Activation. Drug Metabolism and Disposition 35, 419–427 (2007). [DOI] [PubMed] [Google Scholar]
- Yueh M. F. et al. Involvement of the xenobiotic response element (XRE) in ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. Journal of Biological Chemistry 278, 15001–15006 (2003). [DOI] [PubMed] [Google Scholar]
- Yueh M. F., Bonzo J. A. & Tukey R. H. The role of Ah receptor in induction of human UDP-glucuronosyltransferase 1A1. Methods Enzymol 400, 75–91 (2005). [DOI] [PubMed] [Google Scholar]
- Yueh M. F. & Tukey R. H. Nrf2-Keap1 Signaling Pathway Regulates Human UGT1A1 Expression in Vitro and in Transgenic UGT1 Mice. J. Biol. Chem 282, 8749–8758 (2007). [DOI] [PubMed] [Google Scholar]
- Chen S. et al. Intestinal NCoR1, a regulator of epithelial cell maturation, controls neonatal hyperbilirubinemia. Proc Natl Acad Sci USA 114, E1432–E1440 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N. et al. Disruption of the Ugt1 locus in mice resembles human Crigler-Najjar type I disease. J. Biol. Chem 283, 7901–7911 (2008). [DOI] [PubMed] [Google Scholar]
- Liu M. et al. Cadmium and arsenic override NF-kappaB developmental regulation of the intestinal UGT1A1 gene and control of hyperbilirubinemia. Biochem Pharmacol 110–111, 37–46 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson K. G., Contois L. R., Tevosian S. G., Davis R. J. & Paulson K. E. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci U S A 93, 12908–12913 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzardini M., Carelli M., Cabello Porras M. R. & Cantoni L. Mechanisms of endotoxin-induced haem oxygenase mRNA accumulation in mouse liver: synergism by glutathione depletion and protection by N-acetylcysteine. Biochem. J 304 (Pt 2), 477–483 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y. S. et al. Phosphorylation of the nuclear receptor corepressor 1 by protein kinase B switches its corepressor targets in the liver in mice. Hepatology 62, 1606–1618 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum Y. S., Owuor E. D., Kim B. R., Hu R. & Kong A. N. Involvement of Nrf2 and JNK1 in the activation of antioxidant responsive element (ARE) by chemopreventive agent phenethyl isothiocyanate (PEITC). Pharm. Res 20, 1351–1356 (2003). [DOI] [PubMed] [Google Scholar]
- Xu C. et al. Mechanism of action of isothiocyanates: the induction of ARE-regulated genes is associated with activation of ERK and JNK and the phosphorylation and nuclear translocation of Nrf2. Mol. Cancer Ther 5, 1918–1926 (2006). [DOI] [PubMed] [Google Scholar]
- Boyanapalli S. S. et al. Nrf2 knockout attenuates the anti-inflammatory effects of phenethyl isothiocyanate and curcumin. Chem Res Toxicol 27, 2036–2043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal R. & Jaiswal A. K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 93, 14960–14965 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Sherratt P. J. & Pickett C. B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol 43, 233–260 (2003). [DOI] [PubMed] [Google Scholar]
- Tukey R. H., Billings R. E. & Tephly T. R. Separation of oestrone UDP-glucuronyltransferase and p-nitrophenol UDP-glucuronyltransferase activities. Biochem. J 171, 659–663 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Yueh M. F., Evans R. M. & Tukey R. H. The Pregnane-X-receptor controls hepatic glucuronidation during pregnancy and neonatal development in humanized UGT1 Mice. Hepatology 56, 658–667 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. K. et al. Protein kinase A phosphorylates NCoR to enhance its nuclear translocation and repressive function in human prostate cancer cells. J Cell Physiol 228, 1159–1165 (2013). [DOI] [PubMed] [Google Scholar]
- Kawamoto T. et al. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell Biol 19, 6318–6322 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashino T., Ohkubo-Morita H., Yamamoto M., Yoshida T. & Numazawa S. Possible involvement of nuclear factor erythroid 2-related factor 2 in the gene expression of Cyp2b10 and Cyp2a5. Redox Biol 2, 284–288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Glazer A. N. & Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. USA 84, 5918–5922 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme E. H., Zhang J., Schouten E. G. & Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control 12, 887–894 (2001). [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Moore R., Goldsworthy T. L., Negishi M. & Maronpot R. R. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res 64, 7197–7200 (2004). [DOI] [PubMed] [Google Scholar]
- Karin M. & Dhar D. Liver carcinogenesis: from naughty chemicals to soothing fat and the surprising role of NRF2. Carcinogenesis 37, 541–546 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.