Abstract
Background and Objectives
Solar lentigines are commonly found in sun‐exposed areas of the body including hands, neck, or face. This study evaluates the efficacy of an intense pulsed light (IPL) device, with wavelengths between 500 and 635 nm and delivered with a targeted tip, for the treatment of solar lentigines on Japanese skin.
Study Design/Materials and Methods
Forty Japanese patients with solar lentigines received one IPL treatment with a targeted treatment tip that emits wavelengths between 500 and 635 nm and contact cooling. Pulses were delivered through a targeted tip to each lentigo until mild swelling and a gray color were observed. Digital photographs and gray level histogram values were taken pre‐ and post‐treatment, and patient assessments were recorded post‐treatment.
Results
Significant improvement was observed for all patients in digital photographs and mean values of gray level histograms (P < 0.0001). Ninety percent of patients reported satisfaction with the improvement of the treatment area and convenience of the procedure. Complications were minor and transitory, consisting of a slight burning sensation and mild erythema which resolved within 5 hours of treatment. No serious adverse events were observed.
Conclusions
A short‐wavelength IPL, delivered with a targeted tip and contact cooling, offers a highly efficacious treatment for solar lentigines in Japanese skin with minimal downtime and complications. Lasers Surg. Med. 48:30–35, 2016. © 2015 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: Asian patients, gray level histogram values, minimally invasive treatment, photorejuvenation, short‐wavelength intense pulsed light
INTRODUCTION
Non‐ablative photorejuvenation has become an integral procedure in the emerging discipline of laser dermatologic surgery 1. Asian patients with Fitzpatrick skin types III–V are rarely highlighted in publications on cutaneous disorders or cutaneous laser surgery 2. However, Asian patients seek to treat superficial pigmented lesions without complications and post‐treatment downtime. Although the high melanin content confers better photo protection, photo damage in the form of pigmentary disorders is common in Asian skin 3. Furthermore, post‐inflammatory hyperpigmentation (PIH) occurring after cutaneous injury remains a hallmark of skin of color 2, 4.
With increasing use of lasers and light sources to treated pigmentary disorders, prevention, and management of PIH is of great interest in Asian skin, which has a tendency for PIH due to the melanin‐rich epidermis.
Intense pulsed light (IPL) therapy using incoherent broad spectrum light has been reported to be effective for treating superficial pigmented lesions 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15. A multi‐pass, low fluence regimen is required when using large IPL handpieces in order to minimize patient discomfort and risk of side effects, as well as avoid unintended thermal effects in surrounding normal tissue 5.
The IPL device used in this study possesses a small treatment tip to minimize irradiation to surrounding tissue.
In this study, Japanese patients received one treatment using a targeted IPL device with contact cooling and wavelengths ranging from 500 to 635 nm. Treatment efficacy was evaluated using a novel method to objectively quantify color change of treated lentigines.
MATERIALS AND METHODS
Patient Selection
Forty Japanese patients (32 females and 8 males) aged 30–71 years (mean age, 47.3 ± 12.4 years) with Fitzpatrick skin type III–V were enrolled in this study. All patients had visited the Clinica Tanaka Anti‐Aging Center to remove solar lentigines on their cheeks. Patients who had received prior treatment for pigmentary lesions or had skin disease, ephelides, and/or melasma on the cheeks were excluded from this study. Use of skin care products containing skin lightening agents such as tretinoin, hydroquinone, or arubutin was excluded throughout the study. All patients were advised of the potential treatment risks and provided written informed consent for participation in the study prior to initiation of procedures. Subjects received one treatment and were followed for 2–6 months.
Device Description
The IPL device used in this study emits a light spectrum between 500 and 635 nm from a flashlamp to selectively target melanin (AcuTip500; Cutera, Inc., Brisbane, CA) (Fig. 1). Light was delivered to the targeted area with a 6.35 mm targeted tip, with minimum irradiation of the surrounding normal tissue. To avoid any burning sensation and side effects, the sapphire contact cooling tip was set to a fixed temperature of 20°C. The sapphire block was cooled with fluids using thermoelectric coolers circulated by a pump and a cooling system. Pre‐ and parallel cooling of the epidermis was accomplished using the temperature‐controlled sapphire window.
Figure 1.
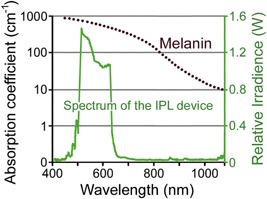
The absorption coefficients of melanin (brown) and relative irradiance of the IPL device (green). Courtesy of Cutera, Inc.
Treatment and Assessments
Before the IPL treatment, the pigmented area of the patient's face was wiped with an alcohol pad to remove any makeup. No topical anesthetics or medications were administered before, during, or after the treatment. Traditionally, clear ultrasound gel is used during IPL treatment to improve light transmission into the skin and aid in epidermal cooling. However, in this study ultrasound gel was not used during treatment. The authors have determined after years of experience that safety and efficacy are not compromised when ultrasound gel is not used with this targeted small tip IPL device.
A single treatment was performed on the pigmented lesion with three to four passes of adjacent pulses ranging in fluence from 10 to 14 J/cm2 and pulse duration of 6.7–9.3 ms. The average interval between passes was 1–2 minutes, and in total, 60–150 pulses were administered to each lentigo. Fluence varied depending on patient skin type and immediate skin reaction observed during treatment. Conventional IPL treatments usually regard a mild darkening of the pigmentation as the clinical end point, whereas in this study, pulses were delivered until a mild swelling and gray color were observed in the lesion (Fig. 2).
Figure 2.
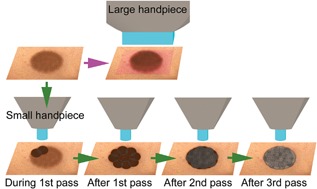
A schematic of IPL treatment for pigmentation with a large handpiece (top) and the IPL treatment with a small handpiece used in this study (bottom).
If the patient reported a severe burning sensation, the time between pulses was extended to adequately cool the skin. Immediately after the procedure, patients were allowed to apply make‐up and instructed to apply sunblock daily.
Digital photographs and gray level histograms, at baseline and 2–6 months post‐treatment, were evaluated as objective assessments. Digital photographs were conducted with a Power Shot G7 camera (Canon Inc., Tokyo, Japan). Best practices were followed to ensure the conditions in both pre‐ and post‐treatment photographs were as identical as possible. The gray level histograms were created using enlarged grayscale images of the treated areas processed with Adobe Photoshop (Adobe, San Jose, CA). The number of pixels at each gray level value from 0 (darkest) to 255 (brightest) were plotted on a histogram. The pre‐ and post‐treatment mean values of the gray level histograms were compared and the difference was tested for statistical significance using Wilcoxon's signed‐rank test (P < 0.05 was set as a cutoff for statistical significance). Data are presented as mean and standard deviation.
Subjective assessments were performed using questionnaire data collected 2–6 months post‐treatment. Patients rated their degree of satisfaction with improvement of the treated lesion, and convenience of the procedure. Scores were based on a 5‐point scale ranging from 0 to 4 (0 = worse; 1 = little satisfaction or not satisfied; 2 = fairly satisfied; 3 = satisfied; and 4 = very satisfied).
RESULTS
Objective assessments evaluated by digital photographs and gray level histograms showed significant improvement in all patients at 2–6 months following one treatment. The mean values of the pre‐ and post‐treatment gray level histograms were 143.7 ± 8.6 and 162.9 ± 9.8, respectively. The mean difference of 19.2 gray level points indicated a clinically and statistically significant improvement of treated solar lentigines at follow‐up (P < 0.0001) (Fig. 3). Forty percent of subjects had greater than 20 points increase in mean gray level value following treatment and demonstrated significant clearing of the treated lesion. Five percent of subjects experienced very significant lesion clearing, defined as greater than 30 points increase in mean gray level value. moderate clearing, or an increase between 10 and 20 gray level points post‐treatment, was observed in 40% of subjects and 15% of subjects showed mild or minimal improvement.
Figure 3.
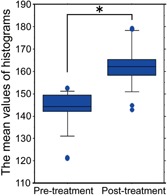
The improvement measured by the values of the gray level histograms at the first pre‐treatment visit and the last post‐treatment visit. A statistically significant difference was observed (P = 0.0001). Data represent the means ± SD. Significant difference is indicated by (*).
Ninety percent of patients reported satisfaction with both improvement of treated lentigines and convenience of the procedure (Fig. 4). The mean satisfaction ratings for improvement of treated lentigines and convenience of the procedure were 3.1 ± 1.0 and 3.4 ± 1.0, respectively. Patients were satisfied with the high efficacy of the treatment as well as the minimal discomfort and side effects. Patients also expressed satisfaction with the short procedure time, and minimal to no post‐treatment downtime. Representative cases are shown in Figures 5, 6, and 7.
Figure 4.
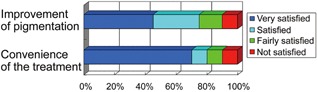
Subjective volunteer assessments were performed using questionnaires. Subjective patient assessments are shown as follows: very satisfied (blue), satisfied (light blue), fairly satisfied (green), and not satisfied (red).
Figure 5.
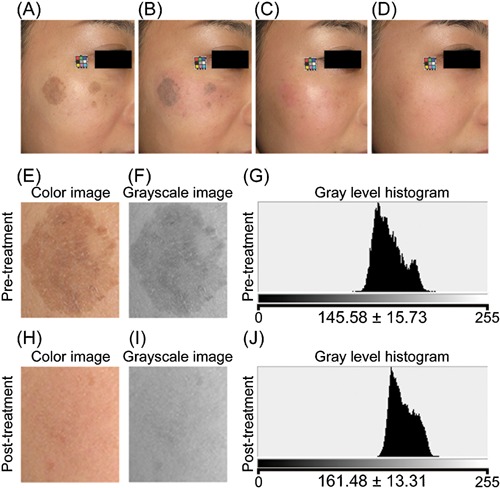
A 30‐year‐old Japanese woman. (A) Pre‐treatment. (B) Just after the treatment. (C) 10 days post‐treatment. (D) 90 days post‐treatment. Four passes at 14 J/cm2 (100 pulses) were applied. Enlarged color image (E, H), grayscale image (F, I), and gray level histogram (G, J) of the treated area pre‐ and post‐treatment, respectively. Significant improvements were observed in digital photographs and gray level histograms.
Figure 6.
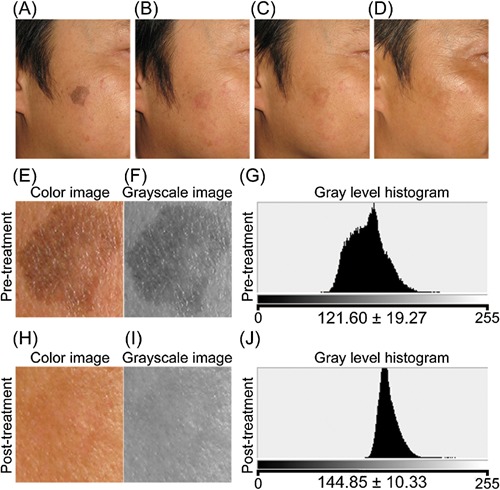
A 47‐year‐old Japanese man. (A) Pre‐treatment. (B) 14 days after the treatment. (C) 60 days post‐treatment. (D) 180 days post‐treatment. Three passes at 10 J/cm2 (60 pulses) were applied. Enlarged color image (E, H), grayscale image (F, I), and gray level histogram (G, J) of the treated area pre‐ and post‐treatment, respectively. Significant improvements were observed in digital photographs and gray level histograms.
Figure 7.
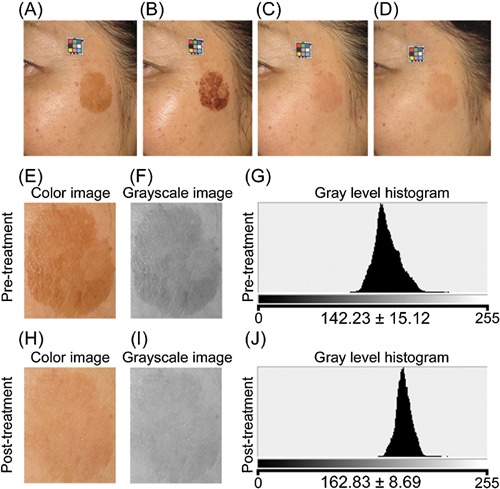
A 59‐year‐old Japanese woman. (A) Pre‐treatment. (B) 4 days after the treatment. (C) 30 days post‐treatment. (D) 60 days post‐treatment. Three passes at 12 J/cm2 (135 pulses) were applied. Enlarged color image (E, H), grayscale image (F, I), and gray level histogram (G, J) of the treated area pre‐ and post‐treatment, respectively. Significant improvements were observed in digital photographs and gray level histograms.
Treated areas slightly darkened immediately after the first pass. after additional passes, the treated areas swelled and turned gray in color. Microcrusts from the treatment lesion desquamated from the skin within 3–14 days. Complications were minor and transitory, with a slight burning sensation reported in four patients and mild erythema in two patients. All side effects resolved within 5 hours of the treatment. No patients reported severe burning sensation during the treatment and no anesthesia was used. Additionally, there were no reports of PIH, hypopigmentation, epidermal burn, scar formation, or lesion recurrence during the study.
DISCUSSION
We previously reported that we can achieve various effects in the targeted tissue with a limited wavelength and contact cooling 16, 17, 18, 19, 20. Many IPL devices emit broad‐spectrum light between 560 and 1,200 nm. Wavelengths between 600–1,300 nm affect a large volume and depth of tissue 21, and wavelengths near 800 nm are used for photodynamic cancer therapy and induction of thermal effects 22, 23, 24. Wavelengths above 1,000 nm are not strongly absorbed by melanin and not ideal for pigmentation treatment. The IPL device used in this study is designed to select for wavelengths between 500 and 635 nm. Shorter wavelengths, such as those within this range, are strongly absorbed by melanin and are highly effective for treating excess pigment.
The mechanism of action for IPL treatment is based on the theory of selective photothermolysis. The controlled absorption of thermal energy by target melanin chromophores within pigmentary lesions leads to their destruction without significant thermal damage to surrounding normal tissue 25. We also theorize IPL treatment may stimulate a rapid differentiation of keratinocytes, accompanied by an upward transfer of melanocytes and subsequent elimination of melanocytes during desquamation of the microcrusts 16.
Microcrusts were observed after the IPL treatment in this study. This microcrust formation seemed to depend on the density and amount of pigment contained in the lesion. A tendency towards a reduction of pigmentation was observed in all subjects after the treatments; however, the amount of pigment removed differed in each subject which seemed to depend on the melanin density and distribution. Although active melanocytes are left behind and pigmentation may recur after the pigmented lesions are temporarily removed as extruded microcrusts 16, recurrence was not observed throughout this study.
Ten percent of study patients were not satisfied with their individual results. The efficacy of IPL therapy for superficial pigment removal is dependent upon the melanin density and distribution. Ephelides and lentigo simplex that are severely pigmented are known to be more effectively treated by IPL, however additional treatment sessions, or a higher energy output, may be required for these patients. Further studies are necessary to determine if higher output or a second treatment may enhance the effects.
In this study we introduced the gray level histogram, a novel method for objective evaluation of post‐treatment color change in solar lentigines. Additional studies are suggested to validate this method for pigmentation measurement. While not yet validated, the gray level histogram could serve as an effective patient communication tool to easily demonstrate measureable lightening of pigmented lesions.
It should be noted that this was a preliminary study based on a fairly small number of patients. We cannot exclude the possibility that intrinsic and extrinsic factors in everyday life may affect the changes demonstrated in this study. Therefore, further studies which include a larger number of patients followed for a longer period of time are recommended to evaluate the treatment effects more exactly.
CONCLUSION
This study demonstrated significant improvement of lentigines and high patient satisfaction in a Japanese population, without severe complications and side effects, after a single intensive targeted IPL treatment using wavelengths between 500 and 635 nm and contact cooling. Furthermore, this non‐ablative approach is convenient for patients and requires almost no downtime.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
Financial Disclosure Statement: This study was conducted without financial support from a third party. We have no relevant financial activities outside the submitted work.
REFERENCES
- 1. Nelson JS, Majaron B, Kelly KM. What is nonablative photorejuvenation of human skin? Semin Cutan Med Surg 2002; 21:238–250. [DOI] [PubMed] [Google Scholar]
- 2. Ho SG, Chan HH. The Asian dermatologic patient: Review of common pigmentary disorders and cutaneous diseases. Am J Clin Dermatol 2009; 10:153–168. [DOI] [PubMed] [Google Scholar]
- 3. Chan HH. Effective and safe use of lasers, light sources, and radiofrequency devices in the clinical management of Asian patients with selected dermatoses. Lasers Surg Med 2005; 37:179–185. [DOI] [PubMed] [Google Scholar]
- 4. Kono T, Manstein D, Chan HH, Nozaki M, Anderson RR. Q‐switched ruby versus long‐pulsed dye laser delivered with compression for treatment of facial lentigines in Asians. Lasers Surg Med 2006; 38:94–97. [DOI] [PubMed] [Google Scholar]
- 5. Willey A, Anderson RR, Azpiazu JL, Bakus AD, Barlow RJ, Dover JS, Garden JM, Kilmer SL, Landa N, Manstein D, Ross EV, Jr , Sadick N, Tanghetti EA, Yaghmai D, Zelickson BD. Complications of laser dermatologic surgery. Lasers Surg Med 2006; 38:1–15. [DOI] [PubMed] [Google Scholar]
- 6. Bitter PH. Noninvasive rejuvenation of photodamaged skin using serial, full‐face intense pulsed light treatments. Dermatol Surg 2000; 26:835–842. [DOI] [PubMed] [Google Scholar]
- 7. Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med 2000; 26:196–200. [DOI] [PubMed] [Google Scholar]
- 8. Negishi K, Wakamatsu S, Kushikata N, Tezuka Y, Kotani Y, Shiba K. Full‐face photorejuvenation of photodamaged skin by intense pulsed light with integrated contact cooling; initial experiences in asian patients. Lasers Surg Med 2002; 30:298–305. [DOI] [PubMed] [Google Scholar]
- 9. Kawada A, Shiraishi H, Asai M, Kameyama H, Sangen Y, Aragane Y, Tezuka T. Clinical improvement of solar lentigines and ephelides with an intense pulsed light source. Dermatol Surg 2002; 28:504–508. [DOI] [PubMed] [Google Scholar]
- 10. Alam M, Hsu TS, Dover JS, Wrone DA, Arndt KA. Nonablative laser and light treatments: Histology and tissue effects‐a review. Lasers Surg Med 2003; 33:30–39. [DOI] [PubMed] [Google Scholar]
- 11. Kligman DE, Zhen Y. Intense pulsed light treatment of photoaged facial skin. Dermatol Surg 2004; 30:1085–1090. [DOI] [PubMed] [Google Scholar]
- 12. Wang CC, Hui CY, Sue YM, Wong WR, Hong HS. Intense pulsed light for the treatment of refractory melasma in Asian persons. Dermatol Surg 2004; 30:1196–1200. [DOI] [PubMed] [Google Scholar]
- 13. Weiss RA, Weiss MA, Beasley KL, Munavalli G. Our approach to non‐ablative treatment of photoaging. Lasers Surg Med 2005; 37:2–8. [DOI] [PubMed] [Google Scholar]
- 14. Dover JS, Bhatia AC, Stewart B, Arndt KA. Topical 5‐aminolevulinic acid combined with intense pulsed light in the treatment of photoaging. Arch Dermatol 2005; 141:1247–1252. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka Y, Tsunemi Y, Kawashima M. Intensive targeted treatment for senile lentigines using intense pulsed light with relatively short wavelengths (500‐635nm) in asian patients. 34th American society for laser medicine and surgery, Phoenix convention center/sheraton Phoenix downtown hotel, Phoenix, Arizona, USA. Lasers Surg Med 2014; 46(4):368. [Google Scholar]
- 16. Yamashita T, Kuwahara T, Gonzalez S, Takahashi M. Non‐invasive visualization of melanin and melanocytes by reflectance‐mode confocal microscopy. J Invest Dermatol 2005; 124:235–240. [DOI] [PubMed] [Google Scholar]
- 17. Tanaka Y, Gale L. Beneficial applications and deleterious effects of near‐infrared from biological and medical points of view. Opt Photonics J 2013; 3(4A):31–39. [Google Scholar]
- 18. Tanaka Y, Tsunemi Y, Kawashima M, Nishida H. The impact of near‐infrared in Plastic Surgery. Plast Surg Int J 2013; Article ID 973073:1–13. [Google Scholar]
- 19. Tanaka Y, Tsunemi Y, Kawashima M, Nishida H, Tatewaki N. Objective assessment of skin tightening using water‐filtered near‐infrared (1000‐1800nm) device with a contact cooling and freezer stored gel in Asians. Clin Cosmet Investig Dermatol 2013; 6:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanaka Y. The impact of near‐infrared radiation in dermatology. Review. World J Dermatol 2012; 1:30–37. [Google Scholar]
- 21. Tanaka Y, Tatewaki N, Nishida H, Eitsuka T, Ikekawa N, Nakayama J. Non‐thermal DNA damage of cancer cells using near‐infrared irradiation. Cancer Sci 2012; 103(8):1467–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol 1981; 77:13–19. [DOI] [PubMed] [Google Scholar]
- 23. Bäumler W, Abels C, Karrer S, Weiss T, Messmann H, Landthaler M, Szeimies RM. Photo‐oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br J Cancer 1999; 80:360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orenstein A, Kostenich G, Kopolovic Y, Babushkina T, Malik Z. Enhancement of ALA‐PDT damage by IR‐induced hyperthermia on a colon carcinoma model. Photochem Photobiol 1999; 69:703–707. [PubMed] [Google Scholar]
- 25. Anderson RR, Parrish JA. Selective photothermolysis: Precise microsurgery by selective absorption of pulsed radiation. Science 1983; 220:524–527. [DOI] [PubMed] [Google Scholar]


