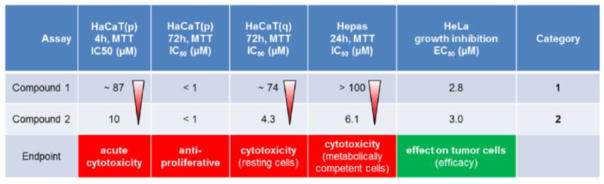
Tab. 2. Illustration of a conventional early in vitro safety assessment of oncology drug candidates.
The test compounds are tested in three different safety screens and the results are compared to the results of the tumor cell line inhibition (Hepas: primary rat hepatocytes; HaCaT: human keratinocytes, non-proliferating quiescent (q) or proliferating (p)). Efficacy of both compounds is in the same range; compound 2 is ranked as critical (category 2) because of its cytotoxicity in a non-proliferating cell at concentrations less than 10-fold above the effective concentrations.