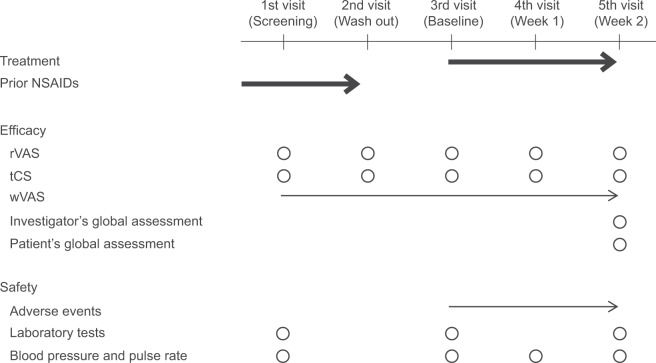
Figure 1.
Study schedule.
Notes: Each patient made weekly visits. At the first visit (screening), the investigator verified the eligibility for study participation. At the second visit (wash out), any ongoing NSAIDs treatment was discontinued to exclude its potential effect on the study. At the third visit (baseline), the investigator verified the conformity with the inclusion criteria and initiated the assigned study treatment. The investigator assessed the efficacy and safety variables at the fourth and fifth visits (week 1 and 2) and completed the observation on the fifth visit unless there were ongoing adverse events to be followed up. Thick arrow, treatment; thin arrow, daily evaluations; circle, evaluations at the time of visit.
Abbreviations: NSAIDs, nonsteroidal anti-inflammatory drugs; rVAS, pain on rising from the chair as determined by visual analogue scale; tCS, total clinical symptoms score; wVAS, pain on walking as determined by visual analog scale.