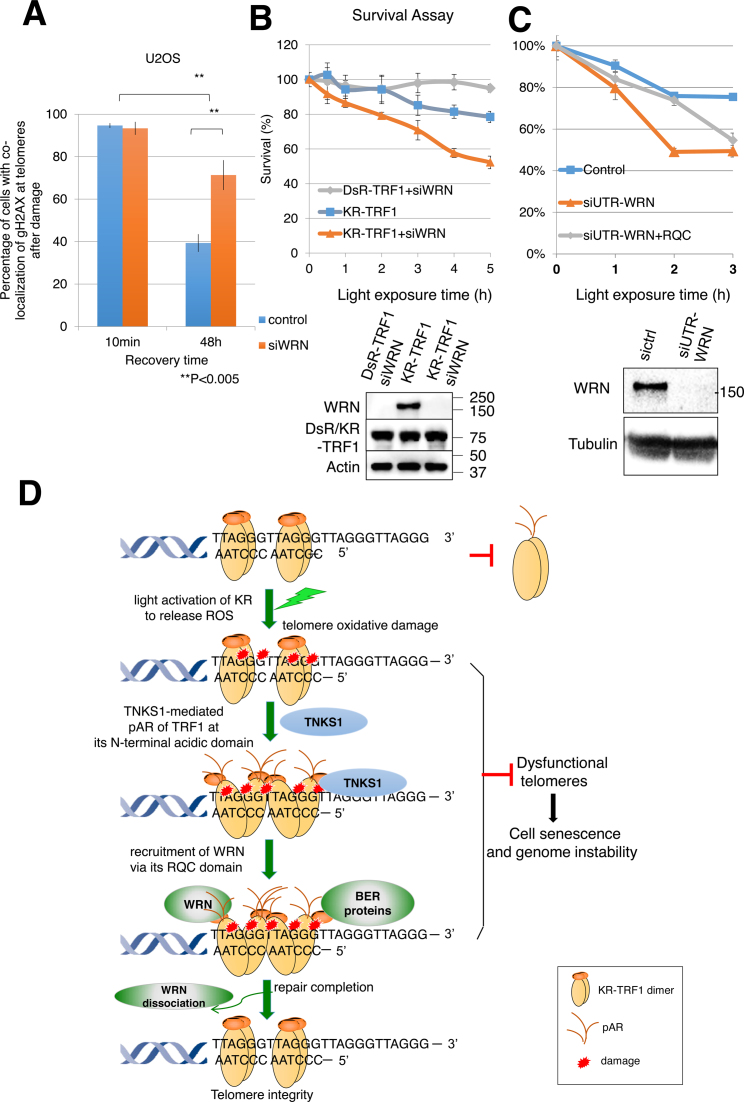
Figure 7.
Suppression of WRN causes delayed repair and increased cell death after oxidative damage at telomeres. (A) Quantification of the percentage of cells showing co-localization of KR-TRF1 and γH2AX with or without siWRN at the indicated recovery time point after 20 min of 15 W SYLVANIA cool white fluorescent bulb light activation of KR. Data are represented as mean ± SEM of three independent experiments with counting 150 cells/time. **P < 0.005 (B and C) Clonogenic survival assay of HeLa cells with stably expressed KR-TRF1 or DsR-TRF1 and treated with or without siWRN (B), siUTR-WRN (C) with or without expression of WRN-RQC. Cells were exposed to cool white fluorescent light for the indicated time. Data are represented as mean ± SEM of three independent experiments. Western blot analysis of WRN knockdown and DsR-TRF1 and KR-TRF1 expression in HeLa cells is shown. (D) A model of recruitment of WRN to oxidative DNA damage at telomeres via interaction with TRF1 upon TNKS1-mediated PARylation. Oxidative DNA damage at telomeres induced a TRF1 conformational change to expose its N-terminal acidic domain. The N-terminal acidic domain of TRF1 will be targeted for PARylation by TNKS1 and then WRN is recruited to sites of DNA damage mediated by interaction between the RQC domain of WRN and N-terminal PARylation of the acidic domain of TRF1. BER factors are recruited independently from WRN. WRN protein dissociates from the sites of damage after repair completion. The function of WRN at telomeres protects genome stability in the face of oxidative DNA damage.