Figure 1.
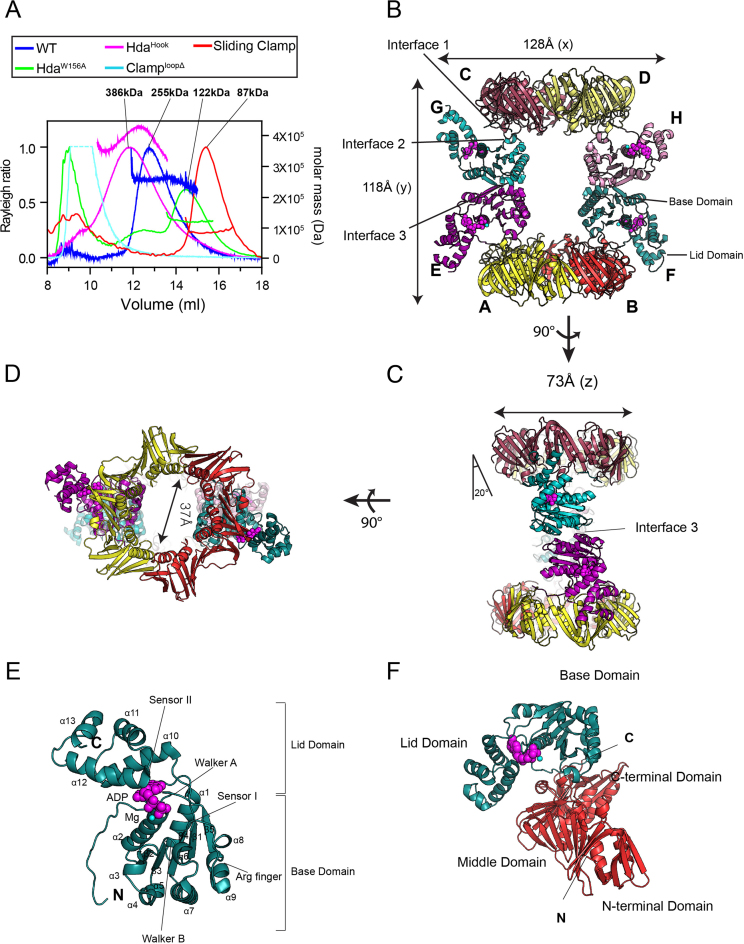
Structure of the Hda–β clamp complex. (A) SEC-MALS analysis of the Hda–β clamp complexes containing wild-type Hda or the indicated mutants, and of the β clamp alone. (B) Structure of the Hda–β clamp octameric complex in ribbon representation. Each β clamp is labeled A, B, C or D. Each Hda molecule is labeled E, F, G or H. ADP (magenta) and magnesium ion (cyan) in each Hda protomer are shown as spheres. Two orthogonal views of the Hda–β clamp complex structure in (B) are shown in (C) and (D). Interface 1, 2 and 3 are indicated in panels B and C. (E) Close-up view of the Hda molecule (chain F). The secondary structures, AAA+ conserved motifs and N- and C-termini of Hda are marked. (F) Heterodimeric arrangement of Hda (chain F) and the β clamp (chain B).