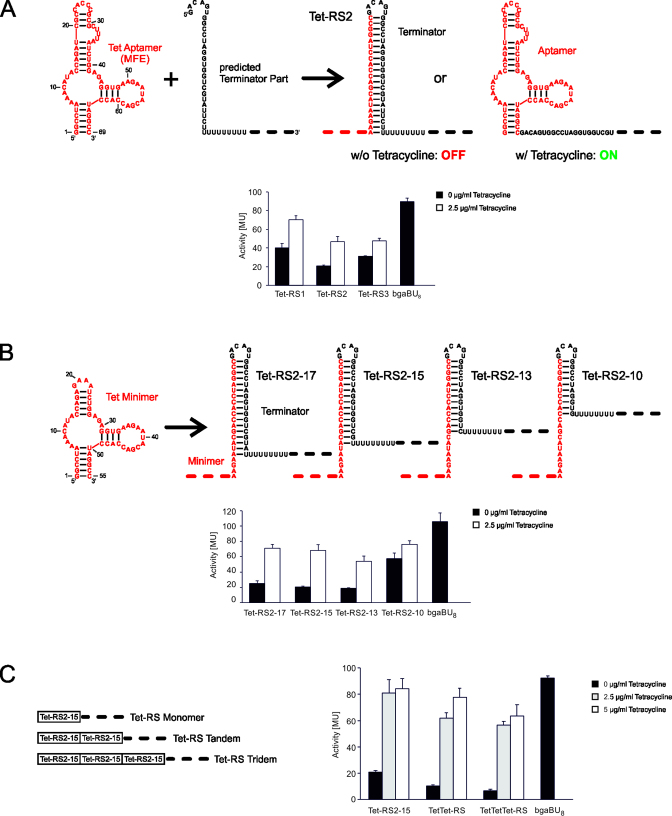
Figure 2.
Activity tests of synthetic tetracycline riboswitches. (A) Design and activity of in silico predicted constructs. In the presence of 2.5 μg/ml tetracycline, expression of β-galactosidase was induced in E. coli, resulting in an increase of Miller units (MU). bgaBU8 represents the appropriate positive control (19,33), consisting of a plasmid expressing the reporter gene under the same promoter but lacking the aptamer and the terminator hairpin. Only the U stretch is present, as this can have an effect on the reporter gene expression. (B) Optimized Tet-RS2 constructs. The terminator hairpin stability of Tet-RS2 was modified by deleting residues from the 3΄-end of the hairpin. In the activity test, these modifications led to an increased response ratio for Tet-RS2-17, Tet-RS2-15 and Tet-RS2-13, while in Tet-RS2-10, the terminator hairpin is too unstable to compete with the aptamer fold. Accordingly, the construct is in a constitutive ON state, independent of tetracycline binding. Furthermore, this behavior is a direct indication that the riboswitch constructs regulate at the level of transcription (19). (C) Serial arrangements of two and three Tet-RS2-15 repeats increase the ligand-dependent responsiveness. The existence of two or three intrinsic terminator elements leads to a reduction of background gene expression in the absence of tetracycline. While the monomeric Tet-RS2-15 shows an absolute activation of 80 MU that is not further increased by a higher tetracycline concentration, tandem and tridem show a somewhat lower absolute response (60–80 MU), with some increase at the higher ligand concentration. As a result, the activation ratios for the tandem and tridem are considerably increased compared to the monomeric riboswitch form.