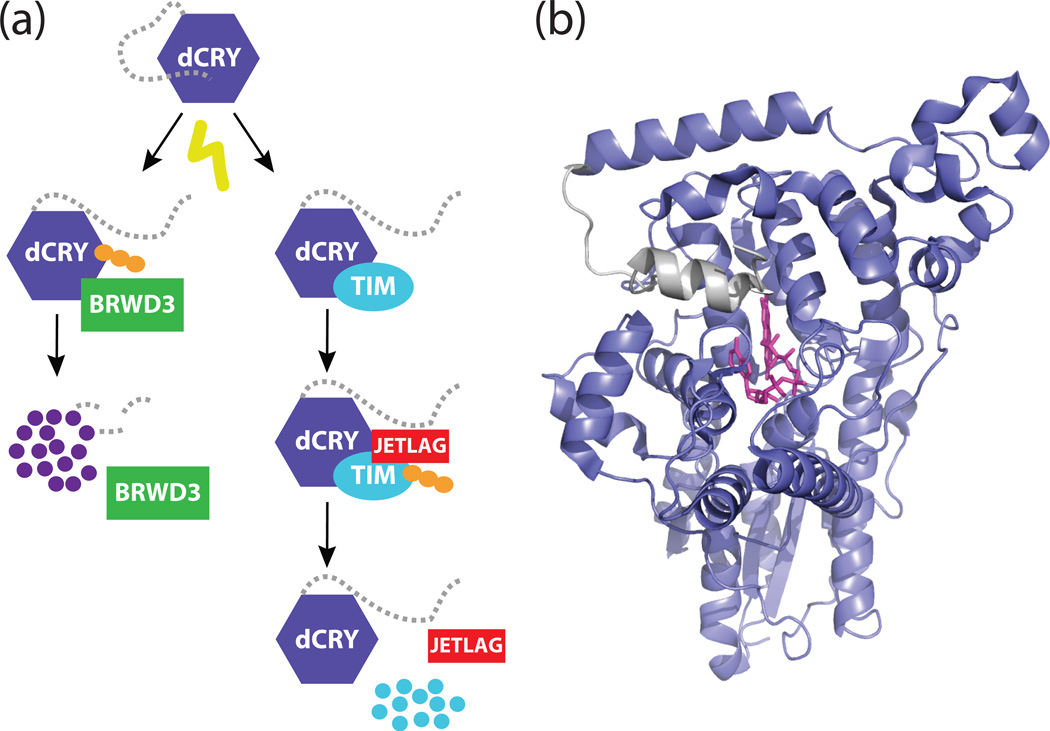
Figure 1. A model for the dCRY C-terminal tail in the Drosophila clock.
(a) Schematic model for the light-responsive degradation of dCRY and TIM. Light stimulates a structural change in dCRY that exposes the binding interface for TIM and JETLAG. This interaction results in the polyubiquitination (depicted as orange circles) and proteasomal degradation of TIM. The same light-induced conformational change in dCRY also renders it sensitive to polyubiquitination by BRWD3. (b) Structure of full-length dCRY (PDB: 4GU5) in the dark-adapted state. A hydrophobic motif in the C-terminal extension (gray) docks onto the PHR domain (purple) in close proximity to the flavin (pink).