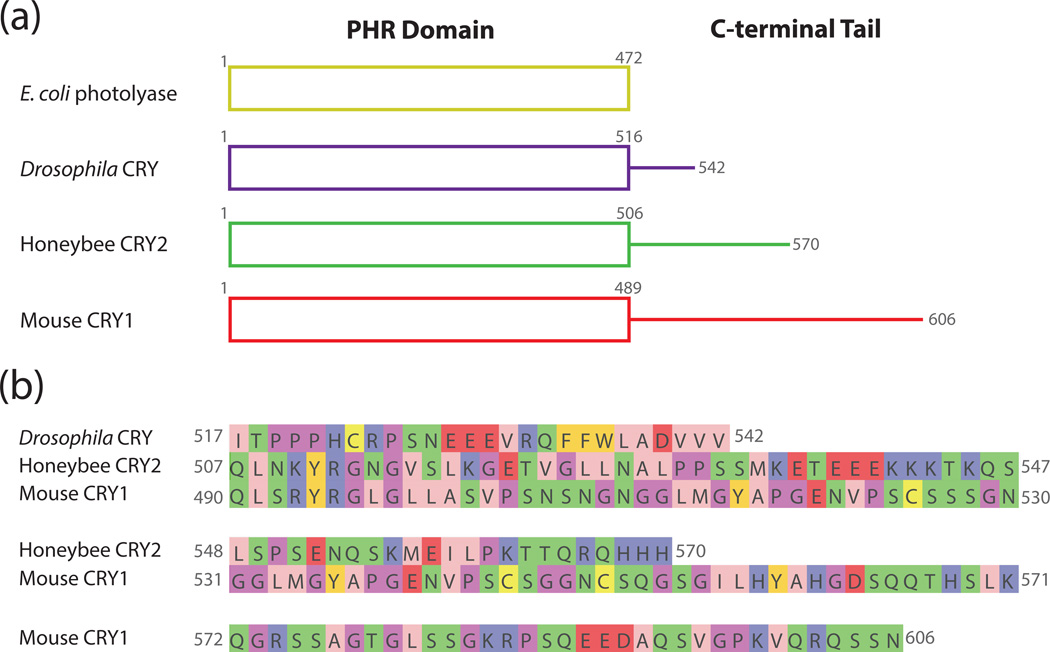
Figure 2. Comparison of C-terminal tail extensions in representative photolyase and cryptochrome proteins.
(a) Schematic representation of the domains present in E. coli photolyase, Drosophila CRY, honeybee CRY2, and mouse CRY1. The overall structure and organization of the PHR domain remains relatively unchanged between the different proteins, but the C-terminal tails vary in length. (b) Sequences of the unstructured C-terminal extensions of Drosophila CRY, honeybee CRY2, and mouse CRY1. Amino acids are colored according to their physicochemical properties using the Jalview Zappo coloring scheme (138): pink, aliphatic/hydrophobic; gold, aromatic; purple, positive; red, negative; green, hydrophilic; light purple, conformationally special; yellow, cysteine.