Abstract
Inflammation has been proposed to play a role in the generation of depressive symptoms. Previously, we demonstrated that patients with major depressive disorder (MDD) have increased plasma levels of the soluble form of the urokinase receptor (suPAR), a marker for low-grade inflammation. The aim of this study was to test the hypothesis that acute exercise would induce inflammatory response characterized by increased suPAR and elucidate whether patients with MDD display altered levels of suPAR in response to acute exercise. A total of 17 patients with MDD and 17 controls were subjected to an exercise challenge. Plasma suPAR (P-suPAR) was analyzed before, during, and after exercise. There was a significantly higher baseline P-suPAR in the patients with MDD, and the dynamic changes of P-suPAR during the exercise were significantly lower in the patients with MDD, compared with the controls. This study supports the hypothesis that an activation of systemic inflammatory processes, measured as elevated P-suPAR, is involved in the pathophysiology of depression. The study concludes that P-suPAR is influenced by acute exercise, most likely due to release from activated neutrophils.
Keywords: Depression, immune activation, inflammatory markers, low-grade inflammation, soluble urokinase plasminogen activator receptor
Introduction
The urokinase-type plasminogen activator receptor (uPAR) is present on immune cells and certain tumor cells.1 It is a membrane-bound receptor that mediates cellular adhesion, differentiation, migration, and proliferation.2 When urokinase (uPA) is bound to uPAR, the receptor is cleaved, and soluble form of the urokinase receptor (suPAR), the soluble form of uPAR, is released. Considering that uPAR only undergoes a small conformational change when it is cleaved, uPAR still has biological functions in the soluble form.3 For example, it has been shown that activated neutrophils release suPAR, and it is hypothesized that this release from neutrophils contributes to the recruitment of monocytes to sites of active inflammation.4,5 Soluble form of the urokinase receptor is known to be a stable marker for low-grade inflammation and plasma suPAR (P-suPAR) concentrations of less than 4.0 ng/mL are considered normal, or termed as low-grade inflammation,6 whereas values above 6 ng/mL may indicate a risk of developing cardiovascular diseases, diabetes, cancer, or may be associated with progressing critical illness, such as sepsis, tuberculosis, and human immunodeficiency virus.7–10 In contrast to most pro-inflammatory and acute response biomarkers, circadian changes in P-suPAR are minimal, and in vitro stability is high.11–13 It is so far unclear whether P-suPAR actually exerts pro-inflammatory actions or just reflects general immune activation.
Enhanced levels of several components of the immune system, eg, interleukin 6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor α (TNF-α) in patients suffering from major depressive disorder (MDD) suggest the involvement of an inflammatory process and a possible physiological link between low-grade inflammation and depression.14–16 A recent study indicates that elevated levels of circulating suPAR in apparently healthy individuals are associated with an increased susceptibility for future use of antidepressants or a clinical depression diagnosis.17 In a previous study, we demonstrated that depressed patients and suicide attempters have increased plasma levels of suPAR.18
By performing a short-time exercise test, the effect of an acute stress situation can be studied. The effect of physical exercise in patients with MDD has been examined in few recent studies. Gustafsson et al19 found that patients with moderate MDD do not have a disturbed peripheral brain-derived neurotropic factor release in response to exercise. Also, Hallberg et al20 investigated the time-dependent changes on short-time exercise of pro-inflammatory cytokines in patients with MDD showing increased levels of TNF-α, IL-6, and interleukin 8 (IL-8), but the general inflammatory markers, such as CRP and serum amyloid protein A, remained constant. Only 2 studies on circulating levels of suPAR related to exercise have been reported. Sanchis-Gomar et al21 showed that serum suPAR levels were not significantly changed in soccer players 12 hours after a game. Mikkelsen et al,22 comparing the levels of suPAR in healthy, normal-weight young and elderly male long-distance runners with untrained, weight-matched men, found that P-suPAR levels were not correlated with the degree of physical activity. The influence on suPAR levels during intensive short-time exercise has not been investigated. The aim of this study was to investigate whether acute exercise has an impact on circulating P-suPAR and P-high-sensitivity CRP levels in medication-free patients with MDD and healthy controls.
Methods
The patient material used in this study originates from Gustafsson et al19 and was approved by the Lund University Medical Ethics Committee. Patients signed a written informed consent.
Subjects
Medication-free patients with MDD (9 women and 8 men) were recruited from the psychiatric clinic at Lund University Hospital. The patients had to meet the diagnostic criteria for moderate or severe MDD according to Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) and scoring of more than 21 on the Montgomery-Åsberg Depression Rating Scale (MADRS) to be included in the study. The controls (9 women and 8 men) were randomly selected from the municipal population register in Lund, Sweden, having no history or current mental or somatic disorder. Patients and controls were sex and age matched ± 5 years. The median ages were as follows: for MDD men 29 (22-54) years, for MDD women 34 (24-53) years, for control men 30 (24-54) years, and for control women 35 (23-54) years. More detailed descriptions about the subjects are previously presented by Gustafsson et al,19 Hallberg et al,20 and Wisen et al.23
Exercise test
The exercise test was performed on a computerized bicycle ergometer (Rødby 380; Siemens Elma, Solna, Sweden) using a standard protocol.24 The exercise test is described in detail by Gustafsson et al,19 Hallberg et al,20 and Wisen et al.23 In short, the test started at a workload of 30 W for women and 50 W for men and was increased stepwise (5 W/30 seconds for women and 5 W/20 seconds for men) until a heart rate of 125 ± 5 beats per minute was attained and persisted at a constant workload for 6 minutes (submaximal workload). Thereafter, the workload was again increased stepwise as described above until exhaustion (maximal workload).25 Blood samples were collected right before the exercise test (baseline), at submaximal and maximal workloads. After the exercise test, patients and controls rested in a sitting position and blood samples were collected after 30 and 60 minutes. All blood samples were collected with a peripheral venous catheter.
Sample handling
Blood samples were collected in EDTA vacuum tubes. The samples were immediately placed on ice and centrifuged at 4°C and 2000g for 10 minutes within 1 hour of collection. Plasma was stored at −80°C until analysis.
Analysis of suPAR
Plasma suPAR concentrations were analyzed using a commercially available enzyme immunoassay (suPARnostic; ViroGates, Copenhagen, Denmark) according to the manufacturer’s instructions. The assay is a double monoclonal antibody sandwich assay that quantifies all circulating forms of suPAR, with a detection limit of 0.1 ng/mL.
Analysis of high-sensitivity
CRP A high-sensitive sandwich enzyme immunoassay (Immundiagnostik AG, Bensheim, Germany) was used for determination of the high-sensitivity CRP concentration in plasma according to the manufacturer’s instructions. The detection limit of the assay was 0.9 ng/mL.
Analysis of albumin
To estimate the exercise-induced hemoconcentration, plasma albumin (P-albumin) was analyzed using a bromocresol green assay (Randox, Crumlin, UK) according to the manufacturer’s instructions. Plasma albumin values were used to estimate hemoconcentration during and after the exercise as follows26:
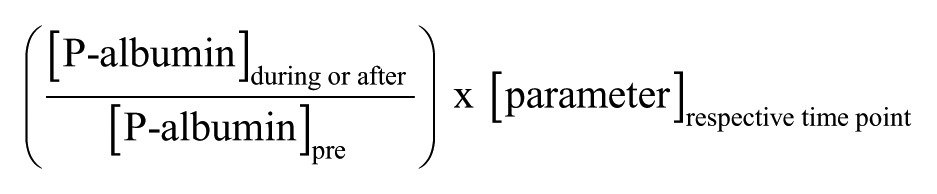 |
where P-albumin is in g/L and “parameter” denotes P-suPAR (ng/mL) or P-high-sensitivity CRP (µg/mL).
Statistical analyses
The baseline differences in the plasma levels of suPAR and high-sensitivity CRP between patients and controls during and after the bicycle challenge were assessed using the Wilcoxon signed rank test and Mann-Whitney U test; statistical significance was considered at *P ⩽ .05; **P ⩽ .01; ***P ⩽ .001. Values are presented as means ± SD.
Results
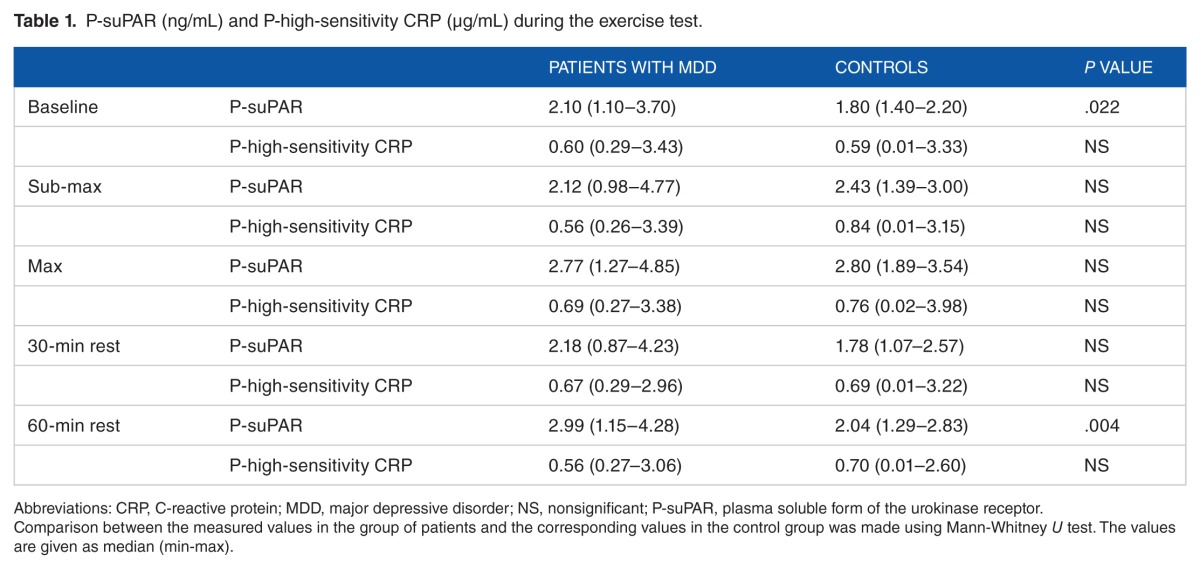
Both patients and controls showed dynamic changes of P-suPAR during the exercise. The increase in P-suPAR levels at maximal workload was greater in controls than in patients (49% vs 27%). Table 1 illustrates median P-suPAR and median P-high-sensitivity CRP during the exercise test for patients and controls. Baseline P-suPAR levels were significantly higher (P = .022) in the patients with MDD compared with the control group (Table 1). The mean baseline P-suPAR level in the control group was 1.8 ± 0.2 ng/mL (the median value was 1.8 ng/mL) and 2.3 ± 0.7 ng/mL in the MDD group (the median value was 2.1 ng/mL). In addition to baseline values, P-suPAR levels in the patients with MDD were also significantly higher after 60-minute rest (P = .004) compared with the control.
Table 1.
P-suPAR (ng/mL) and P-high-sensitivity CRP (µg/mL) during the exercise test.
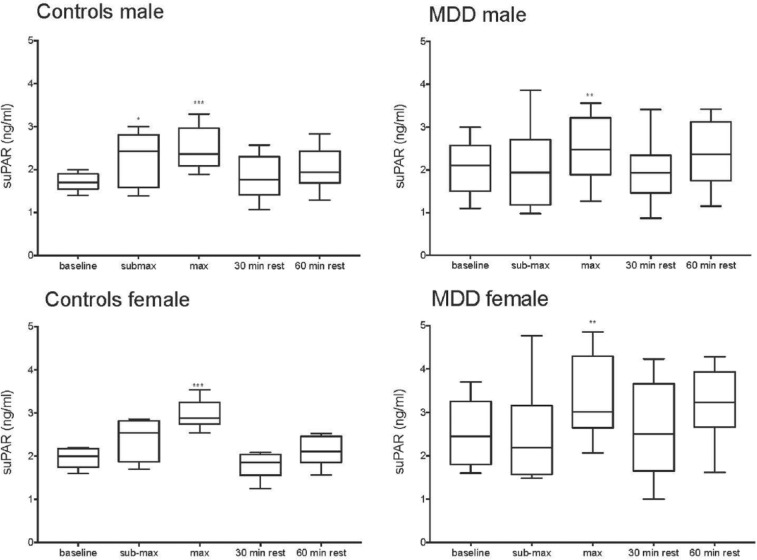
Women and men were also analyzed separately. As depicted in Figure 1, a biphasic pattern in suPAR levels can be seen, in both the patients with MDD and the controls during and after the exercise test. The amount of circulating suPAR after workload decreases after a 30-minute rest, and then increases again, about 10% from baseline values after 60-minute rest in both groups.
Figure 1.
Box plot of P-suPAR (ng/mL) during the exercise test in male patients, male controls, female patients, and female controls. Significant increase (Wilcoxon sign rank test) in comparison with baseline. *P ⩽ .05; **P ⩽ .01; ***P ⩽ .001. MDD indicates major depressive disorder; P-suPAR, plasma soluble form of the urokinase receptor.
P-high-sensitivity CRP levels were not significantly changed during or after the exercise test (Table 1), although a nonsignificant increase in P-high-sensitivity CRP levels in both patients and controls was observed during the exercise, which was normalized during rest. There was no significant correlation between P-suPAR and P-high-sensitivity CRP.
Discussion
In this study, P-suPAR and P-high-sensitivity CRP levels were quantified in medication-free patients with MDD and healthy controls in response to acute exercise. The results clearly indicate that initially, P-suPAR levels were higher in depressed men and women, whereas a significant increase in P-suPAR levels after acute exercise in both depressed patients and controls was observed. Interestingly, a decreased dynamic response to maximal exercise was seen in the patients with MDD compared with the controls which might be related to ongoing inflammatory processes and reduced metabolic activity in the MDD group. P-high-sensitivity CRP levels were not significantly elevated in patients. However, there was a nonsignificant increase in P-high-sensitivity CRP levels at maximal workload in both patients and controls, which was normalized during rest (Table 1). In contrast to most pro-inflammatory and acute response biomarkers, suPAR is recognized as a stable protein with minimal circadian modifications. The results of this study indicate that P-suPAR is influenced by short-time exercise. The changes of circulating suPAR concentrations during exercise cannot be explained by hemoconcentration due to exercise-induced dehydration as the suPAR values are corrected using P-albumin as a reference.
The elevated P-suPAR level, but not P-high-sensitivity CRP levels, in patients with MDD is in accordance with our previous study18 that also showed significantly higher P-suPAR levels in depressed patients but no significant difference in P-high-sensitivity CRP levels between depressed patients and healthy controls. The biomarkers suPAR and CRP may reflect different aspects of low-grade inflammation. Lyngbaek et al27 have shown that CRP mainly reflects inflammation related to adipose tissue, whereas suPAR is linked to endothelial dysfunction and atherosclerosis. In this study, a well-defined group of medication-free patients with MDD were chosen. None of the patients suffered from, eg, melancholic depression or suicidal behavior. This study demonstrates that patients with MDD had significantly higher levels of circulating suPAR before and after 60 minutes of rest after the exercise test. Apparently, by monitoring suPAR levels, as opposed to CRP levels, distinction can be made between controls and patients with MDD, and hence, suPAR is a more compelling marker of low-grade inflammation in depression.
A bout of exercise provokes the appearance of several cytokines in the circulation, including IL-6, IL-8, and IL-10, whereas TNF-α is stimulated only by very intense exercise.28–32 Hallberg et al20 evaluated pro- and anti-inflammatory cytokines after acute exercise using the same clinical setting as this study. No change over time of the classical anti-inflammatory cytokines IL-10 and IL-13 was detected, but the pro-inflammatory cytokines IL-6 and TNF-α increased in depressed patients and controls in response to the exercise test.20 However, IL-6 is considered to have anti-inflammatory properties as well.33,34 It has been suggested that IL-6 released during exercise may exert both regulatory and anti-inflammatory effects,35 considering that the exercise-induced increase in IL-6 is followed by increased circulating levels of well-known anti-inflammatory cytokines such as IL-1 receptor antagonist (IL-1ra) and IL-10.29 It has also been suggested that IL-6 regulates the deployment of neutrophils into the circulatory system.36
The origin of circulating suPAR is yet to be determined, but suPAR is released from monocytes,37 endothelial cells,38 and neutrophils5 on stimulation with certain cytokines. Significant amounts of uPAR, intracellularly stored in neutrophils, can translocate to the plasma membrane following cell activation.5 Acute exercise triggers leukocytosis due to demargination caused by shear stress and catecholamines.39,40 Marginated neutrophils may release circulating suPAR, and therefore, P-suPAR increases in response to acute exercise. Unfortunately, leukocyte particle concentrations were not measured in this study. Sanchis-Gomar et al21 investigated serum suPAR levels in soccer players after a game and concluded that suPAR levels were not influenced by physical exercise. However, they compared suPAR levels before the game and after 12 hours. By then, the leukocyte concentration is likely to be normalized. Moreover, it has been described that a single bout of exercise, such as cycling, results in significantly elevated matrix metal-loproteinase 9 (MMP-9)41 levels, and it is known that the region linking D1 and D2D3 in uPAR is susceptible to cleavage by MMPs.5 Matrix metalloproteinases, including MMP-9, are the major components of neutrophilic tertiary granules. The P-suPAR increase (seen at maximal workload) declines after a short period of rest, but 60 minutes after the exercise was interrupted, P-suPAR was again increased in both patients and controls. It would be of interest if this increase continued during a longer period of rest because that might indicate an upregulated uPAR cleavage. Unfortunately, our study design did not include any later blood samples. We have previously investigated the relationship between suPAR and other inflammatory markers such as CRP, procalcitonin (PCT), IL-6, IL-10, and myeloperoxidase (MPO) in patients with severe sepsis.10 The suPAR level did not correlate with CRP, PCT, IL-6, or IL-10. However, there was a significant correlation between suPAR and MPO in plasma. The weak correlation between suPAR and other inflammatory markers might suggest that suPAR reflects general activation of the immune system rather than exerting inflammatory actions. Correlation between suPAR and MPO might suggest that cleavage of suPAR depends on leukocyte activation, ie, reflects activation of the cellular immune system.
Furthermore, it has been described that acute exercise results in significantly elevated plasma levels of lipopolysaccharides (LPS) from gram-negative bacteria due to an increased gastrointestinal permeability.42 Considering that LPS are able to enhance the release of suPAR,43 endotoxemia may contribute to the increased P-suPAR levels detected 60 minutes after the exercise test (Figure 1).
Although regular physical activity in healthy adults is associated with lower CRP levels,44 acute exercise leads to an increase in CRP levels. For instance, it has been shown that plasma CRP levels dramatically increased in marathon runners,45 in cyclists during the Giro d’Italia,46 and in runners who did participate in an ultradistance running race.47 C-reactive protein is synthesized by the liver in response to IL-6.48 And following an acute-phase stimulus, the plasma concentration of CRP begins to rise after 6 hours and peaks around 48 hours after a single stimulus.49 Hence, the duration of the exercise test in this study was too short to detect any significant CRP induction.
The results of this study support the hypothesis that an activation of systemic inflammatory processes, measured as elevated P-suPAR levels, is involved in the pathophysiology of depression. This study also suggests that maximal exercise induces an inflammatory response characterized by increased suPAR levels. The study design does not reveal the precise origin of circulating suPAR, but the most likely source is activated neutrophils. The physiological significance of this observation is unclear and it is apparent that more data are needed on any potential influence of exercise on suPAR levels and what the implications of changed levels would be. In particular, studies that further investigate uPAR cleavage from leukocytes would be advantageous.
Footnotes
PEER REVIEW: Six peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1167 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) received no financial support for the research, authorship, and/or publication of this article.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
AW, ÅW, and LL conceived and designed the experiments. AG and LO analyzed the data. AG wrote the first draft of the manuscript. LO and LL contributed to the writing of the manuscript. AG, FV, AW, LO, LL, and ÅW agree with manuscript results and conclusions. AG, LL, and LO jointly developed the structure and arguments for the paper, made critical revisions, and approved final version. All authors reviewed and approved the final manuscript.
Disclosures and Ethics
As a requirement of publication, author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
REFERENCES
- 1.Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. doi: 10.3233/DMA-2009-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3:932–943. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 3.Masucci MT, Pedersen N, Blasi F. A soluble, ligand binding mutant of the human urokinase plasminogen activator receptor. J Biol Chem. 1991;266:8655–8658. [PubMed] [Google Scholar]
- 4.Resnati M, Pallavicini I, Wang JM, et al. The fibrinolytic receptor for urokinase activates the G protein-coupled chemotactic receptor FPRL1/LXA4R. Proc Natl Acad Sci U S A. 2002;99:1359–1364. doi: 10.1073/pnas.022652999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pliyev BK. Activated human neutrophils rapidly release the chemotactically active D2D3 form of the urokinase-type plasminogen activator receptor (uPAR/CD87) Mol Cell Biochem. 2009;321:111–122. doi: 10.1007/s11010-008-9925-z. [DOI] [PubMed] [Google Scholar]
- 6.Eugen-Olsen J. suPAR—a future risk marker in bacteremia. J Intern Med. 2011;270:29–31. doi: 10.1111/j.1365-2796.2011.02372.x. [DOI] [PubMed] [Google Scholar]
- 7.Kofoed K, Eugen-Olsen J, Petersen J, Larsen K, Andersen O. Predicting mortality in patients with systemic inflammatory response syndrome: an evaluation of two prognostic models, two soluble receptors, and a macrophage migration inhibitory factor. Eur J Clin Microbiol Infect Dis. 2008;27:375–383. doi: 10.1007/s10096-007-0447-5. [DOI] [PubMed] [Google Scholar]
- 8.Lyngbaek S, Marott JL, Sehestedt T, et al. Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int J Cardiol. 2013;167:2904–2911. doi: 10.1016/j.ijcard.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Eugen-Olsen J, Andersen O, Linneberg A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson A, Ljunggren L, Bodelsson M, Berkestedt I. The prognostic value of suPAR compared to other inflammatory markers in patients with severe sepsis. Biomark Insights. 2012;7:39–44. doi: 10.4137/BMI.S9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen O, Eugen-Olsen J, Kofoed K, Iversen J, Haugaard SB. Soluble urokinase plasminogen activator receptor is a marker of dysmetabolism in HIV-infected patients receiving highly active antiretroviral therapy. J Med Virol. 2008;80:209–216. doi: 10.1002/jmv.21114. [DOI] [PubMed] [Google Scholar]
- 12.Florquin S, van den Berg JG, Olszyna DP, et al. Release of urokinase plasmino-gen activator receptor during urosepsis and endotoxemia. Kidney Int. 2001;59:2054–2061. doi: 10.1046/j.1523-1755.2001.00719.x. [DOI] [PubMed] [Google Scholar]
- 13.Sier CF, Sidenius N, Mariani A, et al. Presence of urokinase-type plasminogen activator receptor in urine of cancer patients and its possible clinical relevance. Lab Invest. 1999;79:717–722. [PubMed] [Google Scholar]
- 14.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasco JA, Nicholson GC, Williams LJ, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197:372–377. doi: 10.1192/bjp.bp.109.076430. [DOI] [PubMed] [Google Scholar]
- 16.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 17.Haastrup E, Grau K, Eugen-Olsen J, Thorball C, Kessing LV, Ullum H. Soluble urokinase plasminogen activator receptor as a marker for use of antidepressants. PLoS ONE. 2014;9:e110555. doi: 10.1371/journal.pone.0110555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ventorp F, Gustafsson A, Traskman-Bendz L, Westrin A, Ljunggren L. Increased soluble urokinase-type plasminogen activator receptor (suPAR) levels in plasma of suicide attempters. PLoS ONE. 2015;10:e0140052. doi: 10.1371/journal.pone.0140052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gustafsson G, Lira CM, Johansson J, et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Res. 2009;169:244–248. doi: 10.1016/j.psychres.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Hallberg L, Janelidze S, Engstrom G, Wisén AGM, Westrin Å, Brundin L. Exercise-induced release of cytokines in patients with major depressive disorder. J Affect Disord. 2010;126:262–267. doi: 10.1016/j.jad.2010.02.133. [DOI] [PubMed] [Google Scholar]
- 21.Sanchis-Gomar F, Bonaguri C, Pareja-Galeano H, et al. Effects of acute exercise and allopurinol administration on soluble urokinase plasminogen activator receptor (suPAR) Clin Lab. 2013;59:207–210. doi: 10.7754/clin.lab.2012.120728. [DOI] [PubMed] [Google Scholar]
- 22.Mikkelsen UR, Couppe C, Karlsen A, et al. Life-long endurance exercise in humans: circulating levels of inflammatory markers and leg muscle size. Mech Ageing Dev. 2013;134:531–540. doi: 10.1016/j.mad.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Wisen AG, Ekberg K, Wohlfart B, Ekman R, Westrin A. Plasma ANP and BNP during exercise in patients with major depressive disorder and in healthy controls. J Affect Disord. 2011;129:371–375. doi: 10.1016/j.jad.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Atterhog JH, Jonsson B, Samuelsson R. Exercise testing: a prospective study of complication rates. Am Heart J. 1979;98:572–579. doi: 10.1016/0002-8703(79)90282-5. [DOI] [PubMed] [Google Scholar]
- 25.Wisen AG, Wohlfart B. Determination of both the time constant of vO2 and DeltavO2/DeltaW from a single incremental exercise test: validation and repeatability. Clin Physiol Funct Imaging. 2004;24:257–265. doi: 10.1111/j.1475-097X.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 26.Alis R, Sanchis-Gomar F, Primo-Carrau C, et al. Hemoconcentration induced by exercise: revisiting the Dill and Costill equation. Scand J Med Sci Sports. 2015;25:e630–e637. doi: 10.1111/sms.12393. [DOI] [PubMed] [Google Scholar]
- 27.Lyngbaek S, Sehestedt T, Marott JL, et al. CRP and suPAR are differently related to anthropometry and subclinical organ damage. Int J Cardiol. 2013;167:781–785. doi: 10.1016/j.ijcard.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 28.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 29.Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Gleeson M, Bishop NC. The T cell and NK cell immune response to exercise. Ann Transplant. 2005;10:43–48. [PubMed] [Google Scholar]
- 31.Pedersen BK, Steensberg A, Fischer C, et al. The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proc Nutr Soc. 2004;63:263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- 32.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 33.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 34.Masli S, Turpie B. Anti-inflammatory effects of tumour necrosis factor (TNF)-alpha are mediated via TNF-R2 (p75) in tolerogenic transforming growth factor-beta-treated antigen-presenting cells. Immunology. 2009;127:62–72. doi: 10.1111/j.1365-2567.2008.02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen BK, Akerstrom TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007;103:1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 36.Yamada M, Suzuki K, Kudo S, et al. IL-6 after exercise may play a role in neutrophil mobilization into the circulation. J Appl Physiol (1985) 2002;92:1789–1794. doi: 10.1152/japplphysiol.00629.2001. [DOI] [PubMed] [Google Scholar]
- 37.Sitrin RG, Todd RF, 3rd, Mizukami IF, Gross TJ, Shollenberger SB, Gyetko MR. Cytokine-specific regulation of urokinase receptor (CD87) expression by U937 mononuclear phagocytes. Blood. 1994;84:1268–1275. [PubMed] [Google Scholar]
- 38.Chavakis T, Willuweit AK, Lupu F, Preissner KT, Kanse SM. Release of soluble urokinase receptor from vascular cells. Thromb Haemost. 2001;86:686–693. [PubMed] [Google Scholar]
- 39.Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 40.Peake J, Wilson G, Hordern M, et al. Changes in neutrophil surface receptor expression, degranulation, and respiratory burst activity after moderate- and high-intensity exercise. J Appl Physiol (1985) 2004;97:612–618. doi: 10.1152/japplphysiol.01331.2003. [DOI] [PubMed] [Google Scholar]
- 41.Reihmane D, Jurka A, Tretjakovs P. The relationship between maximal exercise-induced increases in serum IL-6, MPO and MMP-9 concentrations. Scand J Immunol. 2012;76:188–192. doi: 10.1111/j.1365-3083.2012.02720.x. [DOI] [PubMed] [Google Scholar]
- 42.Ashton T, Young IS, Davison GW, et al. Exercise-induced endotoxemia: the effect of ascorbic acid supplementation. Free Radic Biol Med. 2003;35:284–291. doi: 10.1016/s0891-5849(03)00309-5. [DOI] [PubMed] [Google Scholar]
- 43.Ostrowski SR, Plomgaard P, Fischer CP, et al. Interleukin-6 infusion during human endotoxaemia inhibits in vitro release of the urokinase receptor from peripheral blood mononuclear cells. Scand J Immunol. 2005;61:197–206. doi: 10.1111/j.0300-9475.2005.01547.x. [DOI] [PubMed] [Google Scholar]
- 44.Plaisance EP, Grandjean PW. Physical activity and high-sensitivity C-reactive protein. Sports Med. 2006;36:443–458. doi: 10.2165/00007256-200636050-00006. [DOI] [PubMed] [Google Scholar]
- 45.Weight LM, Alexander D, Jacobs P. Strenuous exercise: analogous to the acute-phase response? Clin Sci (Lond). 1991. 81:677–683. doi: 10.1042/cs0810677. [DOI] [PubMed] [Google Scholar]
- 46.Corsetti R, Lombardi G, Lanteri P, Colombini A, Graziani R, Banfi G. Haematological and iron metabolism parameters in professional cyclists during the Giro d’Italia 3-weeks stage race. Clin Chem Lab Med. 2012;50:949–956. doi: 10.1515/cclm-2011-0857. [DOI] [PubMed] [Google Scholar]
- 47.Margeli A, Skenderi K, Tsironi M, et al. Dramatic elevations of interleukin-6 and acute-phase reactants in athletes participating in the ultradistance foot race spartathlon: severe systemic inflammation and lipid and lipoprotein changes in protracted exercise. J Clin Endocrinol Metab. 2005;90:3914–3918. doi: 10.1210/jc.2004-2346. [DOI] [PubMed] [Google Scholar]
- 48.Hirschfield GM, Pepys MB. C-reactive protein and cardiovascular disease: new insights from an old molecule. QJM. 2003;96:793–807. doi: 10.1093/qjmed/hcg134. [DOI] [PubMed] [Google Scholar]
- 49.Kushner I, Broder ML, Karp D. Control of the acute phase response. Serum C-reactive protein kinetics after acute myocardial infarction. J Clin Invest. 1978;61:235–242. doi: 10.1172/JCI108932. [DOI] [PMC free article] [PubMed] [Google Scholar]