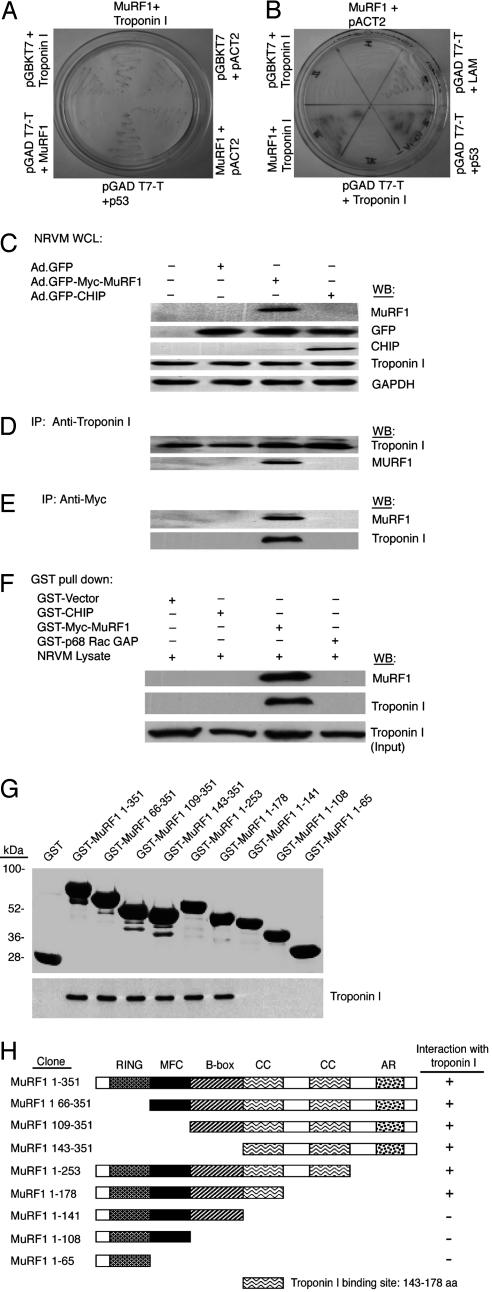
Fig. 1.
MuRF1 interacts with cardiac troponin I in vivo and in vitro.(A and B) Troponin I was retrieved as one of the potential binding partners for MuRF1 in a two-hybrid screen. To reconfirm this binding, a wheel assay was performed by using medium-stringency β-gal filter assays (A) and high-stringency X-α-gal in-gel assays (B). (C–E) To confirm the above interaction in vivo, NRVM were infected with or without Ad.GFP, Ad.GFP-Myc-MuRF1, or Ad.GFP-CHIP at 2 moi. (C) After 24 h, cells were lysed and whole-cell lysates (WCL) were immunoblotted (WB) with anti-Myc (to detect MuRF1), anti-GFP, anti-CHIP, anti-troponin I, or anti-GAPDH as indicated. The cell lysates were immunoprecipitated with anti-troponin I (D) or anti-Myc (E) Abs followed by Western blotting with anti-Myc or anti-troponin I Abs. (F) To confirm the specificity of the interaction between MuRF1 and troponin I, GST-fusion proteins GST-Myc-MuRF1 and negative controls (GST alone, GST-CHIP, and GST-p68RacGAP) were incubated with NVRM lysates that express endogenous troponin I. The protein complexes were subjected to SDS/PAGE and immunoblotted with anti-Myc or anti-troponin I. (G) The region of MuRF1 involved in binding to troponin I was analyzed in pull-down assays. (Upper) GST-MuRF1 fusion proteins were analyzed by blotting with GST Ab. (Lower) The ability of the various MuRF1 fusion proteins to bind to HA-tagged troponin I was analyzed by blotting with HA Ab. (H) Schematic representations of MuRF1 residues that bind to troponin I are indicated. RING, RING domain; MFC, MuRF family conserved region; B-box, B-box domain domain; CC, coiled-coil domains; AR, acidic region.